Synthetic Opioids: Considerations for the Class-Wide Scheduling of Fentanyl-Related Substances
Fast Facts
In 2018, substances related to fentanyl—a very potent synthetic opioid—were temporarily classified as "Schedule I." This designated them as illicit drugs with high abuse potential and no medical use.
This classification has likely affected research, law enforcement, and more. For example, a Schedule I classification may discourage criminal activity, but it can also make it harder for researchers to study a drug.
There are a few ways to classify fentanyl-related drugs once the temporary classification expires in May 2021. For each option, we identified possible tradeoffs (such as complicating law enforcement or making research easier).
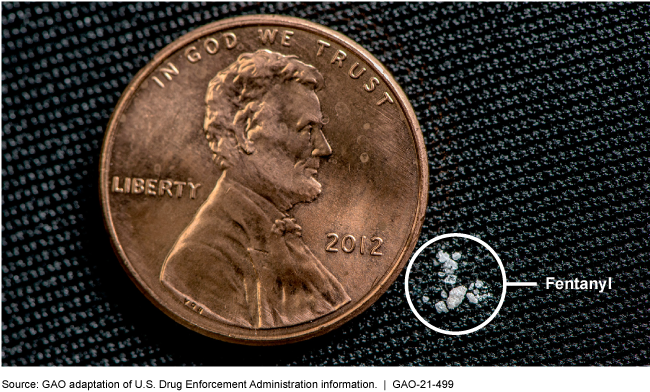
Comparison of a potentially lethal dose of fentanyl to a U.S. penny
Highlights
What GAO Found
In 2018, the Drug Enforcement Administration (DEA) temporarily classified fentanyl-related substances as Schedule I under the Controlled Substances Act, based on their chemical structure, designating them illicit drugs with high abuse potential and no medical use. GAO identified possible considerations for scheduling decisions when the temporary classification expires on May 6, 2021.
Allow the temporary scheduling order to expire. Without the temporary scheduling order, DEA could individually schedule specific fentanyl substances or use the analogue provisions in the Controlled Substances Act to prosecute cases involving unscheduled substances.
- Individually scheduling substances would require evidence of abuse potential and no accepted medical use to classify each substance as Schedule I. This reduces the likelihood of misclassifying substances, as may occur in class-wide scheduling, according to some federal officials.
- Federal law enforcement officials expressed concern that individual scheduling or prosecuting cases as analogues would not be sufficient to deter the creation of new, potentially dangerous substances.
Schedule as a class without modifications. The current temporary scheduling order could be made permanent, such as through legislative scheduling.
- Some substances with unknown potential medical uses or unknown abuse risk could be included in Schedule I.
- Law enforcement officials said that class-wide scheduling has reduced incentives to make new and existing fentanyl substances. GAO's analysis showed a reduction in law enforcement encounters with fentanyl analogues not scheduled individually, but did not determine the cause.
- DEA has approved all 28 researchers who applied to study fentanyl-related substances since 2018, but researchers identified a variety of challenges with research on Schedule I substances, including the time required to obtain approval to conduct such research.
- Civil rights and criminal justice stakeholders cited concerns that class-wide scheduling could result in convictions for substances that may not be harmful and lengthy sentences for trace amounts of fentanyl-related substances and exacerbate racial disparities in federal sentencing.
Legislatively schedule as a class with modifications. Fentanyl-related substances could be legislatively scheduled with modifications to the temporary scheduling order. Potential modifications include those recommended by an interagency workgroup convened by the Office of National Drug Control Policy (ONDCP), such as removing barriers to research and streamlining the process for removing substances from Schedule I if they are discovered to have no abuse potential.
This is a public version of a sensitive report GAO issued in April 2021. Information on China's class-wide scheduling that DOJ deemed sensitive has been omitted from this report.
Why GAO Did This Study
Fentanyl-related substances are powerful synthetic opioids that can be significantly more potent than morphine. The number of deaths from fentanyl-related substances is unknown, but the Centers for Disease Control and Prevention reports that there were more than 50,000 deaths involving all synthetic opioids in the 12-month period ending July 2020.
The Temporary Reauthorization and Study of the Emergency Scheduling of Fentanyl Analogues Act included a provision for GAO to study the classification of fentanyl-related substances and considerations for future scheduling. GAO focused on effects related to drug classification, research, and federal law enforcement, and effects from the classification of similar substances in China.
GAO analyzed documents, data, and statements from federal agencies, such as the Departments of Homeland Security (DHS), Health and Human Services (HHS), Justice (DOJ), and State; ONDCP; and Federal Public and Community Defenders. GAO also interviewed representatives of research, professional, state and local law enforcement, civil rights and criminal justice, industry, and international organizations.
In written comments, ONDCP noted the report identifies the issues to be considered for scheduling. DOJ and the Federal Public and Community Defenders commented on the report's emphasis on different stakeholder perspectives. GAO addressed these comments as appropriate and incorporated technical comments from ONDCP, HHS, DHS, and DOJ.
For more information, contact Alyssa M. Hundrup at 202-512-7114 or hundrupa@gao.gov, Triana McNeil at (202) 512-8777 or McNeilT@gao.gov or KimberlyM.Gianopoulos at (202) 512-8612 or GianopoulosK@gao.gov.
