Food Safety: FDA and USDA Could Strengthen Existing Efforts to Prepare for Oversight of Cell-Cultured Meat
Fast Facts
Soon, people will likely be able to buy cell-cultured meat. To make it, food animals are biopsied and their cells banked, or stored, for use. Then the meat is grown from the cells, harvested, and made into food products. Specifics on the process and the composition of the final product aren’t publicly available.
The FDA and USDA are responsible for food safety and have started oversight work on cell-cultured meat. But they haven’t followed all leading practices for interagency collaboration. Improving how they work together can help them use their resources more efficiently.
Our recommendations address this and other issues we found.
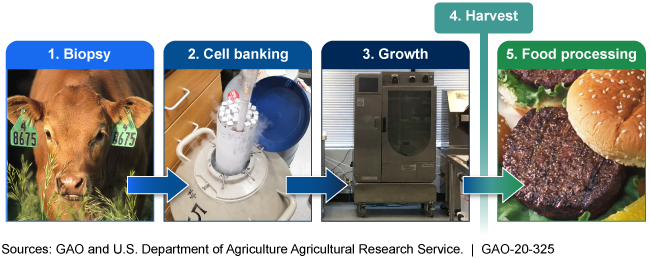
Illustration of the 5 phases of cell-cultured meat production are biopsy, cell banking, growth, harvest, and food process
Highlights
What GAO Found
General information about the process of making cell-cultured meat—food products grown from the cells of livestock, poultry, and seafood—is available. However, no company is commercially producing cell-cultured meat. Specific information about the technology being used, eventual commercial production methods, and composition of the final products is not yet known. The general process contains five phases: biopsy, cell banking, growth, harvest, and food processing (see figure). The technology and methods to be used for commercial production are still in development, and producers, regulators, and consumers do not have clarity about many specifics about the process and final product. For example, it is unclear whether production methods and products will use or contain genetically-engineered cells or medications such as antibiotics.
The Five Phases of Cell-Cultured Meat Production and Federal Oversight Responsibility
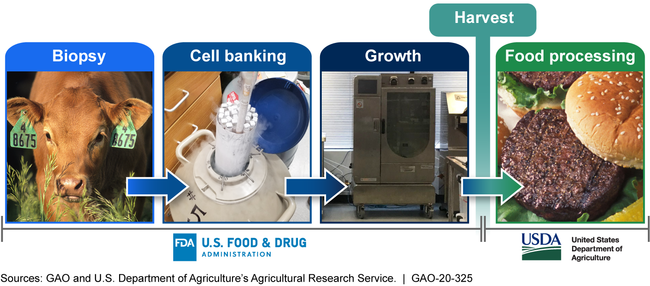
The Food and Drug Administration (FDA) and U.S. Department of Agriculture (USDA) have begun collaborating on regulatory oversight of cell-cultured meat. For example, in 2019, the agencies signed an interagency agreement and created three working groups to carry out the terms of the agreement. However, the agreement and working groups could more fully incorporate practices to enhance and sustain collaboration, such as defining outcomes. For example, the agreement identifies the development of labeling principles as an outcome, but does not describe how the agencies will track and monitor progress toward this outcome, and the working groups identify a lead agency but not members' roles. Also, agency officials said they decided FDA would oversee cell-cultured seafood other than catfish, but they have not formally announced or documented this decision. Developing and updating written guidance and agreements is also a leading practice for interagency collaboration. By fully incorporating leading practices into their efforts to collaborate, the agencies could minimize potential overlap and fragmentation, use resources in a more efficient manner, and better ensure the public and other key stakeholders have clarity about the agencies' oversight responsibilities.
Why GAO Did This Study
Multiple firms have produced cell-cultured meat as part of their research and development. These products appear likely to become available to consumers in coming years. FDA and USDA are the primary agencies responsible for overseeing the safety of the nation's food supply. However, some stakeholders have expressed concern about the agencies' oversight of cell-cultured meat amidst a fragmented federal food safety oversight system.
GAO was asked to review federal oversight of cell-cultured meat. This report (1) describes what is known about methods for commercially producing cell-cultured meat, and (2) examines the extent to which FDA and USDA are collaborating to provide regulatory oversight of cell-cultured meat. GAO conducted a literature review; reviewed documentation from FDA, USDA, and stakeholder groups; analyzed public comments submitted to the agencies; compared agency efforts with leading practices for interagency collaboration; and conducted site visits to selected cell-cultured meat firms.
Recommendations
GAO recommends that FDA and USDA more fully incorporate leading practices for effective collaboration in the agencies' interagency agreement. FDA and USDA partially concurred and indicated a willingness to incorporate these practices in a more detailed agreement, which would also meet the intent of the recommendations. The agencies concurred with the four other recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration | The Commissioner of the Food and Drug Administration, in coordination with the Secretary of Agriculture, should more fully incorporate the seven leading practices for effective collaboration in the agencies' interagency agreement for the joint oversight of cell-cultured meat. (Recommendation 1) |
FDA and USDA partially concurred with this recommendation. FDA stated that it concurred with the intent of incorporating the seven leading practices into the interagency agreement, and both agencies said that they are open to incorporating the practices into their development of the structure for joint oversight of cell-cultured meat. However, the agencies stated that they did not agree to revise the agreement at this time. FDA and USDA stated that the agreement is a general framework and that incorporating the leading practices would constitute an inappropriate level of detail. Instead, the agencies stated that they believe it would be most valuable to incorporate the leading practices into a more detailed joint framework or standard operating procedure they plan to issue. We appreciate the agencies' willingness to incorporate the leading practices for effective collaboration into their efforts. The March 2019 interagency agreement states that the agencies have the ability to modify it as needed and will review the agreement every 3 years to determine whether they should modify or terminate it. Therefore, the agencies were due to revisit the agreement in March 2022, if not sooner. Regarding the agencies' concern that incorporating the leading practices in the interagency agreement would add an inappropriate level of detail, we note that, as we state in our report, the existing agreement already partially incorporates each of the seven leading practices. We continue to believe that FDA and USDA should more fully incorporate the seven leading practices for effective collaboration into their interagency agreement for the joint oversight of cell-cultured meat. Developing a more detailed joint framework or standard operating procedure in accordance with the existing interagency agreement that incorporates those leading practices would meet the intent of our recommendation to improve the effectiveness of the agencies' collaboration. As of December 2025, USDA and FDA had drafted a revised interagency agreement that was pending review and approval by the two agencies. We will review the revised agreement once it is finalized to see if it is responsive to our recommendation.
|
| Department of Agriculture | The Secretary of Agriculture, in coordination with the Commissioner of the Food and Drug Administration, should more fully incorporate the seven leading practices for effective collaboration in the agencies' interagency agreement for the joint oversight of cell-cultured meat. (Recommendation 2) |
FDA and USDA partially concurred with this recommendation. FDA stated that it concurred with the intent of incorporating the seven leading practices into the interagency agreement, and both agencies said that they are open to incorporating the practices into their development of the structure for joint oversight of cell-cultured meat. However, the agencies stated that they did not agree to revise the agreement at this time. FDA and USDA stated that the agreement is a general framework and that incorporating the leading practices would constitute an inappropriate level of detail. Instead, the agencies stated that they believe it would be most valuable to incorporate the leading practices into a more detailed joint framework or standard operating procedure they plan to issue. We appreciate the agencies' willingness to incorporate the leading practices for effective collaboration into their efforts. The March 2019 interagency agreement states that the agencies have the ability to modify it as needed and will review the agreement every 3 years to determine whether they should modify or terminate it. Therefore, the agencies were due to revisit the agreement in March 2022, if not sooner. Regarding the agencies' concern that incorporating the leading practices in the interagency agreement would add an inappropriate level of detail, we note that, as we state in our report, the existing agreement already partially incorporates each of the seven leading practices. We continue to believe that FDA and USDA should more fully incorporate the seven leading practices for effective collaboration into their interagency agreement for the joint oversight of cell-cultured meat. Developing a more detailed joint framework or standard operating procedure in accordance with the existing interagency agreement that incorporates those leading practices would meet the intent of our recommendation to improve the effectiveness of the agencies' collaboration. As of December 2025, USDA and FDA had drafted a revised interagency agreement that was pending review and approval by the two agencies. We will review the revised agreement once it is finalized to see if it is responsive to our recommendation.
|
| Food and Drug Administration |
Priority Rec.
As the three cell-cultured meat working groups move forward, the Commissioner of the Food and Drug Administration, in coordination with the Secretary of Agriculture, should more fully incorporate the seven leading practices for effective collaboration, such as identifying specific outcomes and a way to monitor and evaluate progress toward outcomes. (Recommendation 3) |
In March 2020, FDA officials agreed with this recommendation to improve the working groups that FDA and USDA created to implement the terms of their interagency agreement on oversight of cell-cultured meat. As of December 2021, FDA and USDA had informally incorporated some of the seven leading principles into the working groups' actions. For example, with regard to the leading practice on identifying specific outcomes, FDA reported that it had developed a system to track all tasks for which the agency is responsible. In addition, both FDA and USDA share their progress and milestones for agency-specific tasks during their recurring joint working group meetings. As of January 2023, FDA and USDA had also demonstrated that the working groups assess and share resources and identify opportunities to leverage their resources across agencies. For example, with regard to the leading practice for Identifying and leveraging resources, when FDA staff were overcommitted given the competing needs of other labeling issues at the agency, USDA staff took on some tasks related to drafting labeling principles. Similarly, FDA leaders also shared information and expertise to support USDA staff efforts to draft a cellular agriculture directive. As a result of FDA actions to more fully incorporate the seven leading practices on collaboration, we are closing this recommendation as implemented.
|
| Department of Agriculture |
Priority Rec.
As the three cell-cultured meat working groups move forward, the Secretary of Agriculture, in coordination with the Commissioner of the Food and Drug Administration, should more fully incorporate the seven leading practices for effective collaboration, such as identifying specific outcomes and a way to monitor and evaluate progress toward outcomes. (Recommendation 4) |
In March 2020, USDA officials agreed with this recommendation to improve the working groups that FDA and USDA created to implement the terms of their interagency agreement on oversight of cell-cultured meat. As of December 2021, FDA and USDA had informally incorporated some of the seven leading principles into the working groups' actions. For example, with regard to the leading practice on identifying specific outcomes, FDA reported that it had developed a system to track all tasks for which the agency is responsible. In addition, both FDA and USDA share their progress and milestones for agency-specific tasks during their recurring joint working group meetings. As of January 2023, FDA and USDA had also demonstrated that the working groups assess and share resources and identify opportunities to leverage their resources across agencies. For example, with regard to the leading practice for Identifying and leveraging resources, when FDA staff were overcommitted given the competing needs of other labeling issues at the agency, USDA staff took on some tasks related to drafting labeling principles. Similarly, FDA leaders also shared information and expertise to support USDA staff efforts to draft a cellular agriculture directive. As a result of USDA actions to more fully incorporate the seven leading practices on collaboration, we are closing this recommendation as implemented.
|
| Food and Drug Administration | The Commissioner of the Food and Drug Administration, in coordination with the Secretary of Agriculture, should clearly document in their interagency agreement, or other publicly available document, which agency will oversee cell-cultured seafood other than catfish. (Recommendation 5) |
In response to this recommendation, USDA and FDA have publicly clarified that FDA will oversee cell-cultured seafood other than catfish (i.e., fish of the order Siluriformes). In 2020, FDA took three actions to clarify this. These included an announcement posted to FDA's website stating that food products for human consumption made from cells of species not subject to USDA jurisdiction (e.g., seafood other than Siluriformes and game meat), and food products for animal consumption will be regulated solely by the FDA. Second, in July 2020 FDA and USDA jointly hosted a webinar entitled, "Overview of FDA and USDA Roles and Responsibilities for Cultured Animal Cell Human and Animal Food Products." The webinar makes clear that FDA will oversee cell-cultured seafood other than catfish. FDA posted a recording of this webinar online for public viewing. Lastly, on October 7, 2020, FDA posted a notice to the Federal Register requesting information from the public on the "Labeling of Foods Comprised of or Containing Cultured Seafood Cells." In this notice, the agency states that FDA will continue to regulate foods comprised of or containing cultured animal cells from species under FDA's jurisdiction, such as seafood species other than Siluriformes fish. Together, these actions satisfy the recommendation so we are closing it as implemented.
|
| Department of Agriculture | The Secretary of Agriculture, in coordination with the Commissioner of the Food and Drug Administration, should clearly document in their interagency agreement, or other publicly available document, which agency will oversee cell-cultured seafood other than catfish. (Recommendation 6) |
In response to this recommendation, USDA and FDA have publicly clarified that FDA will oversee cell-cultured seafood other than catfish (i.e., fish of the order Siluriformes). In 2020, USDA took two actions to clarify this. These included an announcement posted to USDA's website stating that food products for human consumption made from cells of species not subject to USDA jurisdiction (e.g. all seafood other than Siluriformes fish and game meat) and food products for animal consumption will be regulated solely by the FDA. Second, in July 2020 USDA and FDA jointly hosted a webinar entitled, "Overview of FDA and USDA Roles and Responsibilities for Cultured Animal Cell Human and Animal Food Products." The webinar makes clear that FDA will oversee cell-cultured seafood other than catfish. USDA posted a recording of this webinar online for public viewing. Together, these actions satisfy the recommendation so we are closing it as implemented.
|
