Food Safety: Additional Actions Needed to Help FDA's Foreign Offices Ensure Safety of Imported Food
Highlights
What GAO Found
The Food and Drug Administration's (FDA) foreign offices have engaged in a variety of activities since 2010 to help ensure that imported food is safe. Foreign offices reported that building relationships with foreign counterparts and gathering and assessing information were among their top priorities. As directed by the FDA Food Safety Modernization Act (FSMA), foreign offices also inspected foreign food facilities. Under FSMA, FDA is to inspect at least 600 foreign food facilities in 2011 and, for each of the next 5 years, inspect at least twice the number of facilities inspected during the previous year. As shown in the figure below, FDA is not currently keeping pace with the FSMA mandate. FDA officials told GAO that they do not plan to meet the FSMA mandate because of funding, and they question the usefulness of conducting that many inspections. However, FDA has not conducted an analysis to determine whether the number of inspections in the FSMA mandate or the lower number of inspections it is conducting is sufficient to ensure comparable safety of imported and domestic food. Without such an analysis, FDA is not in a position to know what is a sufficient number of foreign inspections and, if appropriate, request a change in the mandate.
FDA Inspections of Foreign Food Facilities Compared with FSMA Mandate
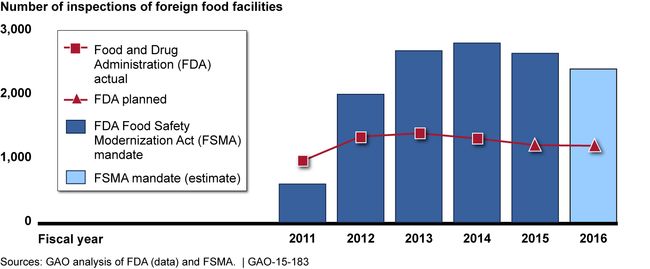
FDA foreign offices cite their contributions to the safety of imported food, but the agency's performance measures do not fully capture these contributions. GAO recommended in 2010 that FDA develop performance measures that can be used to demonstrate the offices' contributions to imported food safety. This recommendation remains valid. FDA has initiated a review to determine how to better reflect the value of the foreign offices in the agency-wide performance systems. Until the offices' contributions are captured, FDA will have less information to effectively measure their progress toward meeting agency goals.
FDA has taken some steps to address recruitment challenges since GAO last reported, but it still does not have a strategic workforce plan. In 2010, GAO recommended that FDA develop such a plan for the foreign offices to help ensure that it recruits and retains staff with the necessary experience and skills. GAO continues to believe that such a plan for the foreign offices is critical to FDA's ability to address staffing challenges, especially since 44 percent of foreign office positions were vacant as of October 2014.
Why GAO Did This Study
FDA has responsibility for ensuring the safety and proper labeling of more than 80 percent of the U.S. food supply, including an increased volume of imported food. Beginning in 2008, FDA established foreign offices to help prevent unsafe products from reaching U.S. borders. In 2010, GAO examined FDA's foreign offices and found that they engaged in a variety of activities relating to food safety but faced challenges due to an increasing workload and other factors. GAO was asked to follow up that report.
This study examines (1) the activities FDA foreign offices have engaged in since 2010 to help ensure the safety of imported food, (2) the extent of the foreign offices' contributions to the safety of imported food, and (3) the extent to which FDA has engaged in workforce planning for its foreign offices. GAO reviewed documentation of foreign office activities and plans, visited offices in China and Mexico, and interviewed agency officials, foreign regulators, and other stakeholders.
Recommendations
GAO recommends that FDA complete an analysis to determine the annual number of foreign food inspections that is sufficient to ensure comparable safety of imported and domestic food. FDA agreed with GAO's recommendation.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration |
Priority Rec.
To help ensure the safety of food imported into the United States, the Commissioner of Food and Drugs should complete an analysis to determine the annual number of foreign food inspections that is sufficient to ensure comparable safety of imported and domestic food. If the inspection numbers from that evaluation are different from the inspection targets mandated in FSMA, FDA should report the results to Congress and recommend appropriate legislative changes. |
In July 2025, FDA stated that given the overlap between activities to address this recommendation and activities to address recommendations from a recent GAO audit on FDA inspections--GAO-25-207571--for efficiency, the agency suggests that progress be reported through updates on the latter audit. As of February 2025, FDA has taken some steps to address this recommendation. In May 2023, FDA completed an analysis that determined 4,695 annual foreign inspections represented an optimal target for ensuring the safety of imported food when combined with other FDA programs and oversight activities. The analysis uses a risk-based approach to prioritize certain foreign facilities for inspections while excluding others for varied reasons. For example, the analysis excludes facilities that selected other countries that are already routinely inspecting as well as facilities that exclusively serve as warehouses. Next, the analysis categorizes the remaining facilities for FDA inspection as either high risk or non-high risk to help ensure FDA prioritizes food facilities that represent the highest risk to U.S consumers. However, the analysis also clearly states that FDA does not have the necessary workforce capacity or resources to meet the identified target of 4,695 foreign inspections annually. Further, in August 2024, FDA officials told us they do not use this annual target in any way when planning FDA's foreign inspection efforts and do not intend to take any further action to implement the approach detailed in the analysis. In addition, FDA has not reported the results of this analysis to Congress or taken steps to recommend appropriate legislative changes to support FDA's foreign inspection efforts. To address the intent of our recommendation, FDA could use the existing 4,695 target and, as we recommended, communicate to Congress regarding the workforce and other resources FDA requires to meet it. Likewise, FDA could instead revisit its May 2023 analysis and use updated information and assumptions to identify a new annual target and communicate it to Congress. By not pursuing either option above, FDA is unable to measure the performance of its overall foreign inspection efforts or assess whether such efforts are achieving intended results-protecting U.S. consumers.
|
