Federal Regulations: Opportunities to Improve the Effectiveness and Transparency of Regulatory and Guidance Practices
Fast Facts
Federal regulations are legally binding, while guidance documents typically are not. Nonetheless, guidance can show an agency's interpretation of regulations and drive its actions.
This testimony shares findings from 2 reports, including:
Selected agencies generally followed guidance development standards but could improve their processes and how they disseminate guidance online
Agencies were less likely to comply with the Congressional Review Act for regulations promulgated during presidential transitions, also called "midnight rulemaking"
We found opportunities to make regulatory and guidance practices more transparent and effective.
Hierarchy of Statutory and Regulatory Authority
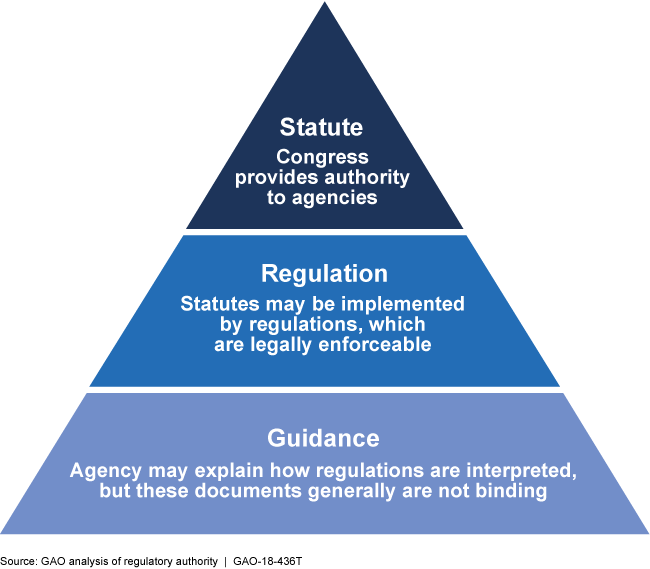
This graphic shows a pyramid with statute as the top layer, regulation as the next, and guidance as the bottom layer
Highlights
What GAO Found
Agencies GAO reviewed—Departments of Agriculture (USDA), Education (Education), Health and Human Services (HHS), and Labor (DOL) did not consistently adhere to Office of Management and Budget (OMB) requirements and internal controls when developing regulatory guidance, as GAO reported in 2015. Unlike regulations, regulatory guidance is not generally legally binding and is subject to different requirements for regulatory oversight. Agencies weighed various factors when they determined whether to issue guidance. The agencies GAO reviewed issued different amounts of guidance for various purposes, such as explaining plans for implementing regulations. Agencies found few of their guidance documents to be “significant,” guidance with a broad and substantial impact on regulated entities. USDA and Education had written procedures for the approval of significant guidance as directed by OMB; DOL's procedures needed updating and to be distributed to appropriate agency officials; HHS did not have any. GAO found that USDA, Education, and DOL consistently applied OMB's requirements for public feedback and access, for example public access to guidance through websites, while HHS did not. Agencies can better ensure consistent application of review processes and public access to significant guidance through better adherence to OMB requirements. GAO also found opportunities for agencies to improve adherence to internal controls for guidance that did not meet OMB's definition of “significant.” For example, most subagencies GAO reviewed did not have written procedures for the production of guidance and about half did not regularly evaluate whether issued guidance was effective and up-to-date. Adherence to these internal controls could promote quality and consistency in guidance development processes.
GAO found that agencies did not consistently comply with the Congressional Review Act (CRA) for regulations promulgated during the 120-day presidential transition periods (September 23 through January 20), as defined by the Presidential Transitions Improvements Act of 2015. GAO reported that during the transition from the end of one presidential administration to the next, the Clinton, Bush, and Obama administrations published on average roughly 2.5 times more economically significant regulations during transition periods than during nontransition periods; increases are typical during transition periods. For these regulations, agencies more frequently provided advanced notice to the public, thus providing the public opportunities to influence the development of these transition period regulations before they were finalized. In their published regulations, agencies generally reported complying with four of five procedural requirements for promulgating regulations during both transition and nontransition periods. Agencies are required to 1) assess the impact of regulations on small entities, 2) minimize the burden that information collections impose on the public, 3) assess the costs and benefits of regulations that include federal mandates, and 4) for certain agencies, obtain direct input from small entities during rulemaking. Also, a fifth requirement, agencies must comply with CRA, which provides Congress an opportunity to review and possibly disapprove regulations before they take effect. Agencies less often complied with CRA, during both transition and nontransition periods. The most common deficiency was agencies' failure to provide Congress the required time to review regulations, which GAO has also identified as a deficiency in previous work.
Why GAO Did This Study
Congress has often asked GAO to evaluate the implementation of procedural and analytical requirements that apply to agencies' rulemaking and guidance processes. The importance of improving the transparency of those processes, including providing public participation and sufficient oversight, is a common theme throughout GAO's body of work on federal regulation.
Based on GAO's prior work, this testimony addresses: (1) the extent to which USDA, Education, HHS, and DOL adhered to OMB requirements and internal controls when developing regulatory guidance, and (2) agencies' compliance with the CRA for regulations promulgated during presidential transitions.
Recommendations
In the April 2015 report on regulatory guidance, GAO made eleven recommendations to USDA, Education, HHS, and DOL to ensure adherence to OMB requirements and applicable elements of internal controls. Three of these recommendations to HHS remain open: 1) to develop written procedures for the approval of significant guidance, 2) strengthen application of internal controls over guidance processes, and 3) improve its website.
In the March 2018 report on rulemaking at the end of presidents' terms, GAO recommended OMB, as part of its regulatory review process, identify economically significant regulations at risk of not complying with the CRA and work with agencies to ensure compliance. OMB staff did not agree or disagree with the recommendation.
