Imported Seafood Safety: FDA and USDA Could Strengthen Efforts to Prevent Unsafe Drug Residues
Fast Facts
Ninety percent of the seafood eaten in the United States is imported, and about half of that is raised on fish farms. Farmers may treat fish with antibiotics and other drugs because these fish can be susceptible to infections. Misuse of drugs can leave residues in seafood that cause health problems for consumers.
We looked at how the two agencies charged with ensuring seafood safety protect against unsafe drug residues, and made five recommendations to strengthen their efforts. For example, agencies could require foreign governments to do more testing for these drug residues.
Major Exporters of Popular Seafood to the United States in 2015
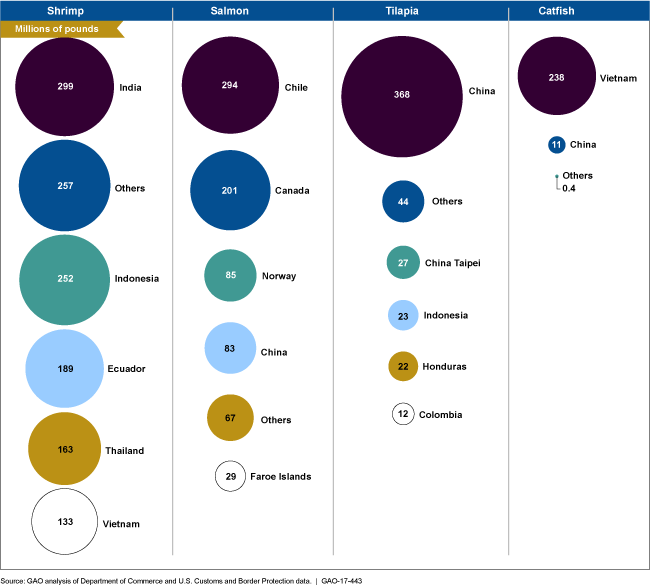
Figure showing countries that export the most shrimp, salmon, tilapia and catfish to United States.
Highlights
What GAO Found
To help ensure the safety of imported seafood from unsafe drug residues, the Food and Drug Administration (FDA) generally depends on the actions of foreign processors and U.S. importers. FDA requires processors and importers to follow its Hazard Analysis and Critical Control Point (HACCP) regulations to identify hazards and the critical control points where the hazards, such as pathogen contamination, are likely to occur and take corrective action. FDA also performs a limited number of (1) inspections of processors and importers each year to ensure HACCP compliance, and (2) tests of imported seafood for contaminants, including unsafe drug residues. FDA could strengthen its efforts to ensure the safety of imported seafood from unsafe drug residues by pursuing agreements with other countries requiring that they test seafood exported to the United States for unsafe drug residues. Under an agency plan, FDA is to coordinate with other countries to increase their capabilities related to the safety of food exported to the United States and better leverage their resources. FDA has used country agreements with respect to pathogen hazards in molluscan shellfish intended for export to the United States. According to FDA officials, it might be worthwhile for the agency to pursue agreements with some countries, but FDA would have to carefully consider a number of factors in determining which countries would be appropriate, which it has not yet done.
In assuming responsibility for inspecting imported catfish, the U.S. Department of Agriculture's (USDA) Food Safety and Inspection Service (FSIS) provided foreign countries and others a transition period—March 1, 2016, through September 1, 2017—before full implementation of its catfish inspection program. Following the transition, countries seeking to continue exporting catfish to the United States are to request equivalence determinations by providing documentation showing that their catfish safety inspection systems are equivalent to the U.S. system. FSIS could strengthen its efforts to ensure the safety of imported catfish. The Agricultural Act of 2014 directs FSIS, in part, to consider the conditions under which catfish are raised, domestically and abroad, but FSIS has not made farm visits a routine part of an equivalence determination. It is not clear how FSIS could consider the conditions under which imported catfish are raised consistent with the act without visiting farms. In addition, during this determination, the agency will already have its inspectors in the foreign country for an on-site audit. FSIS officials generally visit government offices, commercial food processing facilities, and food testing laboratories in a foreign country. Without visiting a sample of farms whose catfish are exported to the United States, FSIS may be missing an opportunity to consider the conditions under which catfish are being raised.
FDA and FSIS took steps to accomplish the transfer of catfish oversight from FDA to FSIS, as called for in the 2014 memorandum of understanding (MOU) that both agencies signed. However, they generally have not coordinated on drug residue testing methods, resulting, in some cases, in differences in drug residue levels used to determine if seafood is unsafe—specifically for unapproved drugs—as called for in the 1984 MOU. Without this coordination, the agencies do not have reasonable assurance that they are consistently protecting consumers from unsafe drug residues.
Why GAO Did This Study
Most seafood consumed in the United States is imported, and about half of it is raised on fish farms. Because farmed seafood is raised in confined areas and susceptible to infections, farmers may use drugs like antibiotics. The use of unapproved drugs or the misuse of approved drugs may result in unsafe residues in seafood that can cause cancer or allergic reactions, according to FDA, which is charged with ensuring the safety of most seafood. Beginning in April 2016, FSIS became responsible for ensuring the safety of imported catfish.
This report examines (1) how FDA helps ensure the safety of imported seafood from unsafe drug residues and ways the agency could strengthen its efforts; (2) how FSIS helps ensure the safety of imported catfish from unsafe drug residues and ways the agency could strengthen its efforts; and (3) the extent to which FDA and FSIS coordinate their oversight efforts. GAO reviewed information from each agency and interviewed agency officials and other key stakeholders.
Recommendations
GAO is making five recommendations, including that FDA pursue agreements with other countries to test seafood exported to the United States and that FSIS visit a sample of fish farms as part of foreign country on-site audits; and that FDA and FSIS coordinate in developing testing methods and corresponding residue levels for imported seafood. FDA agreed with or partially agreed with two; FSIS partially agreed with two and stated it already addresses a third. GAO disagrees and believes the recommendations should be implemented.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration | The Commissioner of FDA should pursue formal agreements with countries exporting seafood to the United States that commit these countries to test for drugs of concern to FDA and the corresponding maximum residue levels (MRLs) that FDA established for these drugs. (Recommendation 1) |
FDA partially agreed with the recommendation. FDA has pursued three formal agreements with countries exporting seafood to the United States, two of which are completed and a third which is underway, according to the agency as of April 2024. The formal regulatory partnership arrangements will further FDA and the foreign country competent seafood safety authorities' commitments to leverage seafood safety controls for product exported to the United States, according to FDA officials. Moreover, according to officials, the agency is prepared to pursue additional agreements. The formal arrangements will reference agreed upon work plans to carry out and share the results of respective countries' testing of aquaculture drugs of concern, according to FDA. Based on FDA's documentation, the agency has pursued formal agreements with countries exporting seafood to the United States and these agreements include sharing information on testing for drugs of concern, which fulfills this recommendation.
|
| Food Safety and Inspection Service | The Administrator of FSIS should ensure that agency staff doing an on-site audit in another country for an equivalence determination visit at least a sample of farms whose catfish are exported to the United States to determine the conditions under which the catfish are being raised, including the drugs being used. (Recommendation 2) |
During the 2018 on-site equivalence determination visits to China, Thailand, and Vietnam, FSIS staff visited farms in each country. FSIS updated its methodology for determining how many and which specific foreign sites FSIS visits during equivalence audits. This methodology now includes visits to aquaculture farms and ponds. According to FSIS, the agency will implement this updated methodology during the audit planning process.
|
| Food Safety and Inspection Service | The Administrator of FSIS should require as part of an equivalence determination that countries exporting catfish to the United States include in their residue monitoring plans the drugs of concern to FSIS and the corresponding maximum residue levels. (Recommendation 3) |
As of April 2024, FSIS identified an alternate method to gather information on drugs of concern and corresponding maximum residue levels, as part of its equivalence determination, from countries exporting catfish to the United States. Specifically, FSIS uses a self reporting tool for countries to submit information as part of the equivalency program. For example, FSIS has found through its review of annual self reporting tool submissions that the three countries allowed to export Siluriformes to the U.S. all maintain residue monitoring programs offering an equivalent level of public health protection as the U.S. FSIS provided copies of three countries' national residue plans and test results from the most recent submission cycle (2020). We reviewed this documentation which appears to support the agency's ability to identify the need for additional information from countries about their residue monitoring plans and maximum residue levels. According to FSIS, if a foreign country's residue monitoring plan does not include drugs that FSIS considers important, the agency will ask that country to explain why those drugs are not included in the plan. According to the agency, as part of the FSIS equivalence program, a foreign country must demonstrate that it either employs the same measures as the U.S., and many foreign countries take this approach, or that the measures that it employs, while different from those of the U.S., provide an equivalent level of public health protection as those of the U.S. While FSIS does not plan to set requirements for foreign countries' residue monitoring plans to include drugs of concern and the corresponding maximum residue levels, FSIS has a process in place to obtain needed information from countries' residue monitoring plans. Therefore, the agency has met the intent of the recommendation through an alternate method.
|
| Food and Drug Administration |
Priority Rec.
The Commissioner of FDA should coordinate and communicate with FSIS in developing drug residue testing methods and corresponding maximum residue levels for imported seafood that may also be applicable to imported catfish. (Recommendation 4) |
FDA agreed with this recommendation. FDA has made all of its methods, single and multi-residue, available to FSIS for use as needed. Both agencies were using the same method for measuring and confirming two unapproved drugs as of February 2022. In addition, FSIS has participated in the Aquaculture Research Working Group's quarterly meetings, in which participants discuss emerging and ongoing research needs in analytical methods development for drug residues in aquaculture products and establishment of limits for their residues. Through this means, FSIS has kept abreast of FDA research work, emerging issues regarding drug residue testing in aquatic animals, and the potential for future collaboration on methods. Additionally, FDA has shared information with FSIS on the types of drugs that are approved for use or suspected of being used in foreign countries that export both Siluriformes (catfish) and other types of seafood to the United States. The agencies stated they will continue to collaborate on establishing, validating, and using sharable methodologies to allow for streamlined and efficient application between agencies when there is a need for new methods. Because the agencies communicate and coordinated on an ongoing and sustained basis, we are closing this recommendation as implemented.
|
| Food Safety and Inspection Service |
Priority Rec.
The Administrator of FSIS should coordinate and communicate with FDA in developing drug residue testing methods and corresponding maximum residue levels for imported catfish that may also be applicable to other imported seafood. (Recommendation 5) |
The agency coordinates with FDA and EPA to carry out the National Residue Program, which entails testing FSIS-regulated products, including catfish, for chemical compounds of public health concern. FSIS officials also participate in FDA's Aquaculture Research Working Group. FSIS makes all its methods publicly available on its website. In addition, FSIS holds monthly coordination and information-sharing meetings through the Interagency Residue Control Group, of which FDA is a member. Moreover, FSIS regularly participates in quarterly interagency meetings, such as the Interagency Surveillance Advisory Team meeting, which provides a forum for agencies such as FDA and FSIS to discuss regulatory residue testing programs as well as to coordinate, communicate, and collaborate on residue testing methods. Since the initial 2017 GAO recommendation, FSIS and FDA have participated in meetings regarding FDA aquaculture research projects and other areas of collaboration with FSIS. These meetings provide FDA with an opportunity to update other agencies on any ongoing research that it is conducting on drug residues involving aquaculture. The meetings also enable FSIS to provide any updates on current aquaculture regulatory work and agency research priority projects involving catfish. For example, there are ongoing discussions between FDA, FSIS, and USDA's Agriculture Research Service focused on improving the analytical method detecting for nitrofurans in catfish. Because of the regular and sustained coordination between the two agencies on drug residue testing methods and maximum residue levels, we are closing this recommendation as implemented.
|
