Science & Tech Spotlight: Medical Use of Psychedelics
Highlights
Why This Matters
Psychedelics can change a person’s perceptions and sense of self, which has promising medical applications. While more research is needed, including on adverse effects, psychedelics may reduce depression and other conditions.
Key Takeaways
Hundreds of clinical trials have examined psychedelics, including for treating PTSD and depression.
Researchers struggle to distinguish genuine effects of psychedelics from the placebo effect.
The medical use of psychedelics also raises questions for policymakers about how to balance potential benefits with research limitations and patient safety.
The Science
What are psychedelics? Psychedelics are hallucinogenic drugs that can temporarily alter a person’s mood and perceptions. Like a class of drugs known as selective serotonin reuptake inhibitors (SSRI), which are often prescribed for mental health care, psychedelics affect serotonin levels (see fig. 1). At certain doses, psychedelics may be used for medical purposes. For example, MDMA (also called ecstasy), LSD, and psilocybin (a hallucinogen found in some mushrooms) have been examined as potential treatments for PTSD and depression.
What is known? Psychedelics work primarily by changing how a person’s brain processes serotonin. This may cause vivid visions, or feelings of insightfulness or connection.
Between 2015 and early 2025, over 340 trials on psychedelics began or were completed, according to ClinicalTrials.gov. For example, one study found that psilocybin reduced depression symptoms more than escitalopram, an SSRI.
While most psychedelics have no federally approved medical uses, several U.S. government agencies have supported such research. For example, in December 2024, the Department of Veterans Affairs planned research to combine MDMA and psychotherapy to treat veterans with PTSD. Several states have also considered or passed legislation allowing medical use of psychedelics.
Psychedelics may have non-life-threatening side effects, such as headaches or vomiting. In some cases, the use of these drugs can lead to safety risks due to impairment, or illicit psychedelics may be contaminated with dangerous substances such as fentanyl. While some research suggests that the use of psychedelics does not typically lead to physical addiction, the adverse effects of these drugs have not been fully studied.
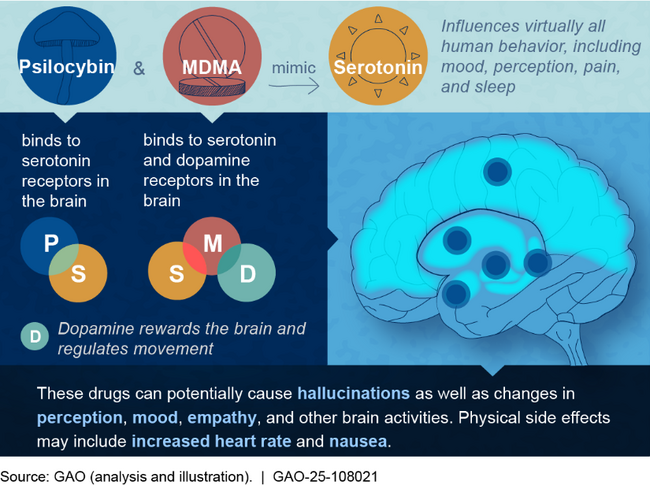
Figure 1: The Science Behind Psilocybin (Psychedelic Mushrooms) and MDMA
What are the knowledge gaps? There is still much to learn about the potential benefits and adverse effects of psychedelics, including how they may be influenced by preexisting conditions or used in combination with psychotherapy. The effects of psychedelics can be difficult to predict and may depend on factors such as the dosage and unique biology of the person receiving the treatment. Moreover, data are limited since many of these clinical trials have been conducted on small groups of participants.
- In blind clinical trials, participants are typically randomly selected to receive either a treatment or a placebo without being told which. This allows researchers to more easily differentiate between the effects of a given treatment and any placebo effect, in which participants who receive a placebo still report a benefit because they expect that the treatment will help.
Because of the distinct effects of psychedelics on recipients, it is difficult to prevent trial participants from knowing whether they received the treatment. This may complicate efforts to design such trials and interpret their findings. Clinical trials have tried to work around this by using an active placebo, such as a low dose of a psychedelic. Alternatively, researchers might compare the treatment with a more typical one, such as an SSRI.
Opportunities
- Mental health. Research has shown that psychedelics, such as psilocybin and MDMA, decrease fear and anxiety, with the potential to positively affect behavior in combination with therapy. For example, a clinical trial with patients with treatment-resistant PTSD demonstrated that combining MDMA and psychotherapy was effective, well-tolerated, and had no major adverse effects years later.
- Pain management. Psychedelics appear to show promise for patients with certain headache disorders and cancer pain. According to a few studies, drugs such as LSD and psilocybin appeared to alter pain perception by activating serotonin receptors and reducing inflammation.
Challenges
- Accessibility. LSD, MDMA, and psilocybin remain illegal at the federal level, categorized as Schedule I substances, which have no federally approved medical uses. To conduct research on these drugs, scientists need to follow several steps. These include obtaining permission from the U.S. Drug Enforcement Administration, finding clinical grade drugs to test, and identifying appropriate spaces in which to test and store these drugs.
- Federal approval. Difficulties associated with conducting large, blind trials of psychedelics have limited researchers’ ability to determine the safety and effectiveness of these drugs, which is required for them to gain approval from the Food and Drug Administration (FDA). FDA approval is generally required before prescription drugs can be marketed for sale in the U.S.
Policy Context And Questions
- How could research be structured to help policymakers better understand any potential benefits and adverse effects of the medical use of psychedelics?
- How could research on medical use of psychedelics be conducted to better reflect requirements for FDA approval?
- What steps could policymakers take to weigh the potential benefits and risks of psychedelics, considering policy implications, resource demands, and patient needs?
Selected References
National Institute on Drug Abuse. “Psychedelic and Dissociative Drugs.” Accessed January 21, 2025. https://nida.nih.gov/research-topics/psychedelic-dissociative-drugs
A. Wem et al., “A Systematic Review of Study Design and Placebo Controls in Psychedelic Research,” Psychedelic Medicine, vol. 2, no. 1 (March 2024).
For more information, contact Karen L. Howard, PhD at (202) 512-6888 or howardk@gao.gov.
