Drug Shortages: HHS Should Implement a Mechanism to Coordinate Its Activities
Fast Facts
Drug shortages are a serious public health problem affecting patient access to care, such as cancer treatment. The COVID-19 pandemic worsened existing drug supply chain issues and shortages are lasting longer.
The Food and Drug Administration aims to prevent and respond to drug shortages, but can't do it alone. Fully addressing shortages will require the Department of Health and Human Services to work across FDA and its other agencies, and with others. Our leading practices for collaboration—e.g., defining common outcomes—could help.
We recommended that HHS determine how to formally collaborate on drug shortages, using our leading practices.
Highlights
What GAO Found
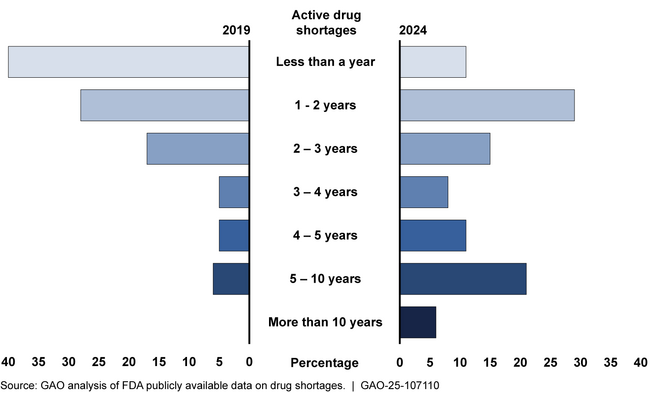
Drug shortages are a serious public health concern that can adversely affect patients by delaying or limiting access to care. Challenges with the Food and Drug Administration’s (FDA) oversight of medical products, including drug shortages, led to its inclusion on GAO’s High-Risk List. As of July 31, 2024, there were 102 drug shortages being tracked by FDA. Since the start of the COVID-19 pandemic in 2020, the number of new drug shortages reported each year has generally decreased, although drug shortages are lasting longer. The types of drugs in shortage generally continued pre-pandemic trends. For example, shortages most commonly affect sterile injectable drugs that are critical to hospital care and cancer treatment. Further, the pandemic exacerbated existing supply chain vulnerabilities that underlie shortages. For example, shortages of a drug used to prevent blood clotting during surgeries were exacerbated by demand increases during the pandemic. This affected patient care in life-threatening situations, according to a patient advocacy group.
Duration of Drug Shortages from December 29, 2019, and July 31, 2024
FDA, within the Department of Health and Human Services (HHS), is responsible for tracking and addressing drug shortages in the U.S. As such, FDA has several efforts underway to improve how it addresses shortages. For example, FDA has taken steps to develop data analytic tools to help its staff better analyze drug supply chain information and potentially predict possible drug supply disruptions. FDA also started developing an effort to encourage manufacturers to invest in more mature quality systems, as quality issues underlie many shortages.
Drug shortages are a multifaceted issue that require a collaborative governmental approach to address them, according to FDA, Congress, and others. However, HHS did not have a coordinating structure across the department to oversee its responses and strategies. This limited the capability of HHS to mitigate and respond to shortages and strengthen supply chain resilience, according to HHS. In November 2023, President Biden announced a coordinator position within HHS to strengthen medical product supply chains and address related shortages. HHS took steps to establish this position. For example, it appointed an acting coordinator that developed a task force that included representatives from agencies across HHS.
In responding to GAO's draft report, HHS notified GAO that the coordinator position will end in May 2025, because funding originally designated for these activities will expire. This will leave HHS without a mechanism for coordinating the department's drug shortage activities. The department stated that the current administration had not indicated how it will direct and coordinate supply chain activities moving forward.
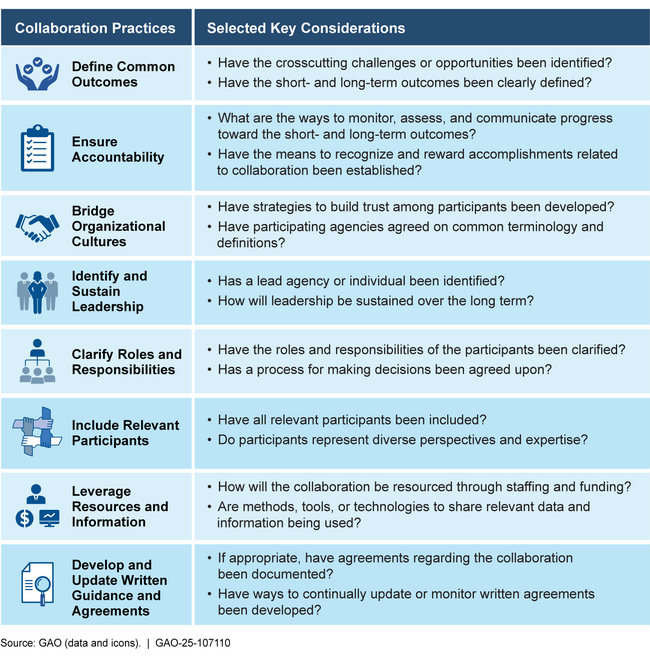
Given the longstanding nature of this critical public health issue, it is important that HHS identify and implement a mechanism to coordinate its drug shortage activities and collaborate with other federal stakeholders. Once a mechanism is identified, taking into consideration GAO's leading practices for interagency collaboration when developing that mechanism will be critical to ensuring HHS can effectively address drug shortages.
GAO's Leading Interagency Collaboration Practices and Selected Key Considerations
Why GAO Did This Study
Drug shortages arise from a variety of factors that contribute to supply chain vulnerabilities, such as lack of incentives to produce less profitable drugs and to invest in manufacturing quality. While FDA helps respond to and prevent drug shortages, it cannot address some of the economic factors affecting the supply chain like other agencies can, such as by purchasing drugs or funding certain manufacturing.
The CARES Act includes a provision for GAO to report on the federal pandemic response. This report (1) describes the trends in drug shortages since the start of the COVID-19 pandemic, (2) describes steps FDA is taking to improve its drug shortage response and prevention efforts, and (3) examines the status of the Supply Chain Resilience and Shortages Coordinator position.
GAO analyzed FDA data from 2017 to 2024 to obtain information on drug shortages; identified new efforts that FDA had underway since GAO last reported on the issue in 2016; reviewed relevant FDA documents and guidance; and interviewed officials from HHS and a nongeneralizable sample of 15 organizations representing entities affected by drug shortages, such as manufacturers, patients, and providers.
Recommendations
GAO is making two recommendations: that the Secretary of Health and Human Services (1) identify and implement a mechanism to formally coordinate its drug shortage activities and collaborate with other federal stakeholders, and (2) ensure this mechanism takes GAO leading practices for collaboration into consideration.
When HHS was provided a draft of this report for review, GAO recommended that the coordinator document how GAO’s leading practices will be used for coordinating across the federal government to help address drug shortages. In written comments, HHS stated that it did not concur with GAO’s recommendation, as the coordinator position and its associated actions would be ending in May 2025. Consistent with this new information, GAO revised its recommendation, as stated above.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Department of Health and Human Services | The Secretary of Health and Human Services should identify and implement a mechanism to formally coordinate HHS strategies to address drug shortages and collaborate with other federal stakeholders, as needed. (Recommendation 1) |
When we confirm what actions the agency has taken in response to this recommendation, we will provide updated information.
|
| Department of Health and Human Services | The Secretary of Health and Human Services should ensure this mechanism takes into consideration GAO's Leading Practices to Enhance Interagency Collaboration and Address Crosscutting Challenges as it carries out its activities to address drug shortages. (Recommendation 2) |
When we confirm what actions the agency has taken in response to this recommendation, we will provide updated information.
|
