Drug Safety: FDA Should Implement Strategies to Retain Its Inspection Workforce
Fast Facts
The Food and Drug Administration paused many drug manufacturer inspections during the COVID-19 pandemic. Inspections help ensure that the drugs Americans rely on are safe.
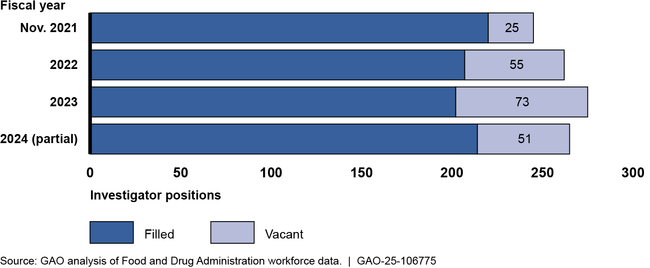
Since resuming inspections, FDA has struggled to retain staff. From Nov. 2021 to June 2024, the vacancy rate among investigators who inspect foreign and domestic manufacturers jumped from 9% to 16%—leading to fewer inspections.
FDA said concerns with travel, pay, training, workload, and work-life balance contribute to turnover. For example, investigators can travel up to 75% of the time.
We recommended developing a plan that balances inspection needs with addressing turnover.

Highlights
What GAO Found
After disruptions during the COVID-19 pandemic in 2020, the Food and Drug Administration (FDA) had largely resumed conducting in-person inspections of foreign and domestic drug manufacturers by March 2022. FDA data show it conducted 621 foreign and 444 domestic inspections in fiscal year 2023, although there were 36 percent fewer than in fiscal year 2019. This decrease was due in part to reduced investigator capacity, according to FDA. As of May 2024, FDA had also progressed with the implementation of two pilot programs intended to address challenges unique to foreign inspections: conducting unannounced inspections and utilizing independent interpreters.
FDA expanded the use of alternative tools to maintain oversight of drug manufacturing during the pandemic. FDA has continued to use one tool—relying on inspection reports from trusted foreign regulators—in lieu of conducting inspections. However, FDA's use of other tools—remote assessments of information from manufacturers—has declined and will largely be reserved for more targeted use now that inspections have resumed, according to FDA.
GAO previously reported that as of November 2021, FDA had taken steps to reduce vacancies among its drug inspection workforce. However, since then, investigator attrition has generally outpaced hiring and has resulted in a large number of relatively inexperienced investigators. FDA told GAO this has limited the number of inspections FDA can complete. FDA identified root causes of attrition as the frequency and conditions of travel, pay, insufficient training, heavy workload, and issues of work-life balance. It is implementing action plans to address pay and training. FDA has not yet developed action plans to fully address travel, workload, and work-life balance because potential solutions may not allow FDA to meet its inspection needs. However, the continued loss of experienced investigators is already affecting FDA's ability to meet inspection goals. Therefore, developing and implementing action plans to address these remaining root causes will help FDA maintain the experienced workforce it needs to oversee global drug manufacturing. This will require continued collaboration with leadership and other stakeholders to identify any actions, resources, or new authorities necessary to implement such plans.
FDA Drug Investigator Vacancies, November 2021–June 2024
Why GAO Did This Study
FDA, an agency within the Department of Health and Human Services (HHS), inspects foreign and domestic drug manufacturers as a key oversight tool to ensure the safety and quality of drugs marketed in the U.S. In response to travel disruptions caused by the COVID-19 pandemic, FDA paused many inspections and relied on the use of alternative inspection tools to oversee drug manufacturing.
The Consolidated Appropriations Act, 2023, includes a provision for GAO to report on the status of FDA's foreign drug inspections and its use of alternative tools. This report describes the status of FDA in-person inspections, describes FDA's use of alternative tools, and examines FDA investigator vacancies and the agency's efforts to address them, among other objectives. For this work, GAO examined FDA data and documents and interviewed FDA officials and investigators who conducted inspections and used alternative tools. GAO also reviewed documents from and interviewed six stakeholder groups that represent the major components of the drug manufacturing industry.
Recommendations
GAO recommends that FDA collaborate to develop and implement action plans to address the remaining root causes of investigator attrition that balance inspection needs against the need to retain investigators. HHS agreed with this recommendation.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration |
Priority Rec.
The Commissioner of FDA should ensure that FDA develops and implements action plans that address attrition caused by issues with investigator travel, workload, and work-life balance. In doing so, ORA, CDER, and other relevant stakeholders should collaborate to identify strategies that balance current inspectional needs against the need to retain an experienced workforce and identify any necessary actions, resources, or new authorities. (Recommendation 1) |
HHS agreed with this recommendation. In its comments on the draft report, HHS said that FDA plans to establish a committee that includes representatives from all affected departments to comprehensively address issues related to attrition. This committee will be responsible for developing a detailed action plan with defined timelines and deliverables. This committee will also collaborate with the existing Inspections Oversight Board to consolidate efforts related to attrition and create a holistic strategy. As of January 2025, GAO was awaiting HHS's first update on actions to address this recommendation.
|
