Autism Research and Support Services: Federal Interagency Coordination and Monitoring Efforts Could Be Further Strengthened
Fast Facts
We also published an "Easy Read" version of this report. Easy Read is a way of making written information easier to understand. We published the Easy Read version to make our report more accessible to certain people with autism and other intellectual and developmental disabilities.
To promote the health and well-being of people with autism, the National Institutes of Health helps coordinate the activities of 18 federal agencies—including research on contributing factors and training on how to screen patients for autism.
NIH has followed several key practices to coordinate agency programs and activities but could do more. For example, NIH could track its progress toward goals to help agencies better allocate resources where they are most needed. And NIH could document how its Office of National Autism Coordination checks for unnecessary duplication of federal efforts across agencies.
Our recommendations address this.

Highlights
What GAO Found
The National Institutes of Health (NIH), within the Department of Health and Human Services (HHS), plays a key role in supporting the coordination of autism activities across 18 federal agencies, including the Departments of Defense and Education. For example, NIH manages the Interagency Autism Coordination Committee (IACC), a federal advisory committee composed of federal agencies and public members, through its Office of National Autism Coordination.
GAO found that NIH, in support of the IACC and the National Autism Coordinator, generally followed six of eight key collaboration practices that GAO's prior work has shown can be effective in enhancing and sustaining interagency collaborative efforts among federal entities. For example, NIH has taken steps to bridge organizational cultures by convening meetings of the IACC.
Assessment of the National Institutes of Health's (NIH) Role in Supporting Coordination of Federal Autism Activities Compared with Leading Practices for Interagency Coordination
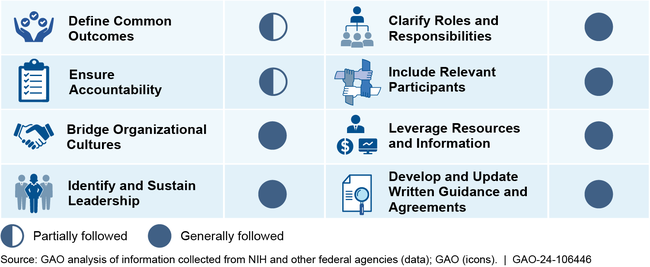
GAO found NIH efforts to support interagency coordination partially followed the remaining two collaboration practices, including ensuring accountability. For example, although IACC strategic plans describe high-level progress made toward autism activities, they generally have not described how progress made relates to goals. NIH officials stated their progress tracking approach is driven by established processes, some of which are required by law. Establishing a clear process for tracking progress would help to determine progress toward IACC's goals and that interagency efforts are effective.
NIH helps ensure federally funded autism activities are not unnecessarily duplicative through various activities, such as holding meetings and through data and information reviews. However, GAO found the processes used by NIH's Office of National Autism Coordination were not documented. For example, NIH does not have written procedures describing the steps these staff should follow when reviewing federal autism research information for potential duplication. Although NIH officials stated that they believe current monitoring processes are sufficient, documenting these procedures will help ensure they are properly designed and executed to provide reasonable assurance that duplication is not occurring.
Why GAO Did This Study
The federal government plays an important role supporting research, programs, and other activities to promote the health and well-being of people with autism. Multiple federal agencies are involved in autism activities. To help coordinate and monitor federal autism activities and to ensure activities are not unnecessarily duplicative, Congress directed the Secretary of Health and Human Services to establish the IACC and designate an official to facilitate coordination and implementation of autism activities, known as the National Autism Coordinator.
GAO was asked to examine coordination and monitoring of federal autism activities. This report examines NIH efforts to (1) help coordinate federal autism activities and (2) monitor autism activities to ensure federal autism activities are not unnecessarily duplicative.
GAO reviewed NIH documents and relevant federal laws; assessed NIH's role in supporting coordination of autism activities against key practices that GAO identified in prior work; and gathered information from 19 federal agencies that conduct autism activities.
Recommendations
GAO is making two recommendations: HHS should (1) develop a process to clearly track and report progress toward IACC goals; and (2) ensure that NIH documents the procedures its Office of National Autism Coordination uses to ensure federal autism activities are not unnecessarily duplicative. HHS concurred with the recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Department of Health and Human Services | The Secretary of Health and Human Services should establish a process for clearly tracking and reporting progress made toward goals established by the IACC for federal autism activities, coordinating with federal partners as appropriate. Such progress tracking should describe where performance is lagging, and why desired results were not achieved, if any. (Recommendation 1) |
HHS agreed with GAO's recommendation and has taken some steps in response to it. In its August 2024 update, HHS noted that it included three tables in the most recent iterations of the portfolio analysis report published in March 2024 and the report to Congress published in December 2023, which agency officials said help to track and report progress made towards goals. For example, one table indicates whether the goals have at least one associated research project ongoing. However, these tables do not describe how IACC goals will be achieved or where performance on goals is lagging. HHS officials said in October 2024 that descriptions of how performance is lagging are provided in the IACC strategic plan, but we found this information is not presented clearly for each IACC goal. In July 2025, HHS reported that it would issue a biennial update to the IACC strategic plan, as required by the Autism Collaboration, Accountability, Research, Education, and Support Act of 2024, that would highlight progress made towards goals established by the IACC and indicate implementation gaps. Further, HHS provided a draft of one chapter to illustrate how HHS plans to incorporate this information into its biennial update. The draft chapter identifies the status of some of the goals the IACC established for federal autism activities, including by providing examples of funded projects and opportunities for continued achievement. HHS officials indicated they plan to include similar information for each chapter's goals in the biennial update, and reiterated this intention in December 2025. Once it is issued, we will review the biennial update to ensure it serves as a mechanism for clearly tracking and reporting progress made toward each IACC goal, including describing where performance is lagging, and why desired results were not achieved.
|
| Department of Health and Human Services | The Secretary of Health and Human Services should ensure that NIH documents the procedures the Office of National Autism Coordination uses, in its support of the IACC and the National Autism Coordinator, to help ensure federal autism activities are not unnecessarily duplicative. Such documentation should describe the roles and responsibilities of different entities, sources of information used, the time frames for conducting analyses, and how outcomes will be reported. (Recommendation 2) |
HHS agreed with GAO's recommendation and, in July 2025, provided finalized standard operating procedures for monitoring autism research and non-research activities for potential unnecessary duplication. These procedures specify the roles and responsibilities of different entities, sources of information used, time frames for conducting analyses, and how outcomes will be reported. With these documented procedures, the Secretary of Health and Human Services will have greater assurance that federal funding supporting autism activities is being used in the most efficient manner.
|
