Clinical Research: FDA Should Evaluate Its Efforts to Recruit and Retain Its Inspection Workforce
Fast Facts
The FDA oversees clinical trials and other research involving human subjects, mostly for drugs trying to get FDA approval. This includes conducting inspections in hospitals and other healthcare settings in the U.S. and abroad.
Recruiting and retaining investigators for these inspections has been a challenge—partly due to low compensation and heavy travel. This has resulted in fewer inspections and a less experienced workforce.
FDA has increased compensation and offered a student loan repayment program, but attrition remains a persistent problem and training new investigators can take up to a year.
Our recommendation addresses this issue.
FDA Biomedical Research Investigator Workforce, FY 2018-2023

Highlights
What GAO Found
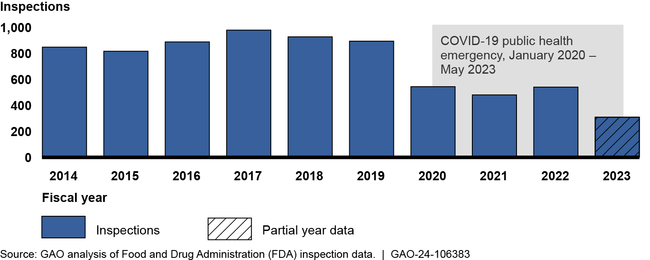
The Food and Drug Administration (FDA) conducts inspections to, among other things, help ensure the quality and integrity of clinical research used to support drugs seeking marketing approval in the U.S. FDA's clinical research inspections peaked in fiscal year 2017 but have since declined. FDA officials attributed this decrease to the COVID-19 pandemic and not having enough investigators.
Number of FDA Clinical Research Inspections Related to Drugs, Fiscal Year 2014 through March 1, 2023
From fiscal years 2012 through 2020, FDA classified 3 percent of clinical research inspections as having serious deficiencies that would warrant regulatory actions. Investigators GAO spoke with were frustrated that problems they identified (e.g., failure to follow research protocols) did not result in more serious classifications. FDA is limited in its ability to cite serious deficiencies for a common type of study supporting generic drugs. Specifically, the regulations for these studies do not include certain requirements for basic study conduct, such as record retention and following study protocols. FDA has started the process of revising these regulations. Having effective requirements will be important to help ensure high-quality research.
FDA has faced challenges recruiting and retaining investigators, resulting in fewer inspections and a less experienced workforce. For example, FDA was unable to complete about 30 percent of one type of common inspection within the requested time frames from fiscal year 2018 through July 2023, according to agency information. FDA officials and the investigators GAO spoke with identified low compensation and high amounts of travel as contributing to these challenges. FDA has taken steps to increase recruitment and retain investigators, such as increased compensation and student loan repayment. The agency recently made progress recruiting new investigators, but attrition has been a persistent problem and it can take new investigators up to a year to independently conduct inspections. Although FDA made progress, the agency has not formally evaluated its efforts to determine their effectiveness. Such an evaluation could help FDA determine whether it is using the most appropriate tools to maintain its workforce. GAO has cited workforce as a concern across multiple FDA programs and sustained attention in this area will be critical.
Why GAO Did This Study
Clinical research—clinical trials and other studies involving human subjects—for drugs seeking FDA approval can occur in the U.S. or overseas. During inspections, FDA goes on-site, such as to hospitals or other health care settings, to examine research protocols and records as well as the entity and facility involved in the research. Challenges in other FDA inspection programs contributed to GAO placing FDA medical product oversight on its High-Risk List in 2009.
GAO was asked to review FDA's inspections of clinical research. This report, among other objectives, describes inspections FDA conducted from fiscal years 2012 through 2023; describes the frequency with which FDA identified serious deficiencies during inspections; and examines FDA's efforts to maintain its investigator workforce. For this work, GAO examined FDA data and documents and interviewed FDA officials. GAO also interviewed 15 out of about 100 investigators, selected to represent diversity among the different investigator positions and tenure with FDA.
Recommendations
GAO is making one recommendation that FDA evaluate its recruitment and retention efforts to determine their effectiveness and incorporate results, as appropriate, to help ensure the agency is using the most appropriate tools to maintain its investigator workforce. The Department of Health and Human Services, of which FDA is a part, agreed with GAO's recommendation.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration | The Commissioner of FDA should evaluate its recruitment and retention efforts for BIMO investigators—such as increased pay, student loan repayment, and other financial incentives—to determine their effectiveness and incorporate results of this evaluation as appropriate to help ensure the agency is using the most appropriate tools to maintain its BIMO investigator workforce. (Recommendation 1) |
FDA concurred with our recommendation. In September 2024, FDA indicated in written comments that it was gathering data to evaluate the effectiveness of these recruitment and retention efforts. It wrote that evaluating these data-such as the effect of increased pay and student loan repayments-will enable FDA to target the most effective methods for recruiting and retaining BIMO investigators, but that some of these efforts may take time to evaluate. In a July 2025 written response, FDA indicated that it was not able to provide a detailed update on actions to address this recommendation based on a range of broader HHS and FDA policy directions that were actively being reviewed and assessed. However, it also indicated that GAO's recommendation would be considered as part of the wider context shaping current discussions. We will continue to monitor FDA's efforts. This recommendation will remain open until FDA provides documentation about how it will incorporate the results of these evaluations into its recruitment and retention efforts.
|
