End-Stage Renal Disease: CMS Plans for Including Phosphate Binders in the Bundled Payment
Fast Facts
Patients experiencing kidney failure rely on dialysis and other treatments for life-sustaining care. The Centers for Medicare & Medicaid Services pays dialysis organizations for the care with a bundled payment that includes dialysis, certain drugs, and other costs like lab work.
Currently "phosphate binders"—drugs commonly used among this population—aren't part of the bundle. CMS plans to add them in 2025, which may require dialysis organizations to expand their capacity to dispense high volumes of the drugs.
CMS plans to assess whether the bundled payment adequately covers the cost of phosphate binders and will monitor the drugs’ use.
Highlights
What GAO Found
Phosphate binders are oral drugs commonly used by Medicare beneficiaries with end-stage renal disease to treat mineral and bone disorder, which may result in weak and brittle bones. Currently, these drugs are paid for separately from the bundled payment that the Centers for Medicare & Medicaid Services (CMS) established for dialysis and most end-stage renal disease-related treatment under Medicare's traditional fee-for-service program. The bundled payment is designed to incentivize efficient care, because dialysis organizations retain the difference if Medicare's bundled payment exceeds the cost of providing services. Congress has delayed until 2025 the inclusion of phosphate binders in the bundled payment. Beginning in 2025, CMS plans to pay for phosphate binders using an add-on payment for at least 2 years. Subsequently, CMS plans to modify the bundled payment to account for the cost and utilization of phosphate binders but has not yet finalized its approach for doing so.
Timing of CMS's Plans for Including Phosphate Binders in End-Stage Renal Disease Bundled Payments
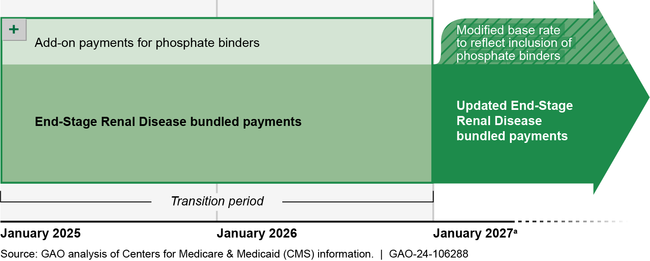
aCMS's proposal is to pay for phosphate binders for at least 2 years using an add-on payment to dialysis organizations. CMS officials said they may extend the transition period to a third year for CMS to collect sufficient claims data to accurately set the payment rate.
When CMS includes phosphate binders in the bundled payment, dialysis organizations will be responsible for dispensing these drugs to beneficiaries. All dialysis organization representatives GAO interviewed anticipate needing to expand their existing capacity to dispense oral drugs. They attributed this need, in part, to the high volume of phosphate binder prescriptions. In addition, these dialysis organization representatives expressed concerns that the modified bundled payment may not fully account for the costs of acquiring, shipping, and dispensing phosphate binders. CMS officials expect that it will be feasible for dialysis organizations to make necessary operational changes. CMS officials said they would assess the adequacy of payments. Agency officials also said they would examine concerns raised by the public through the rulemaking process to ensure continued access to these medications.
CMS plans to monitor beneficiary utilization of phosphate binders and health outcomes related to phosphate binder treatment once these drugs are included in the bundled payment.
Why GAO Did This Study
Dialysis services provide life-sustaining treatment for individuals with end-stage renal disease. In 2021, Medicare covered dialysis treatment under Part B for nearly 332,000 beneficiaries with the disease who were enrolled in Medicare's traditional fee-for-service program. Currently, phosphate binders are covered under Medicare's prescription drug benefit (Part D). In 2020, Medicare Part D plans paid almost $1 billion for these drugs.
The American Taxpayer Relief Act of 2012 includes a provision for GAO to review issues associated with the inclusion of phosphate binders in the bundled payment.
This report describes (1) CMS's plans to include phosphate binders in the bundled payment; (2) dialysis organizations' views on their capacity to dispense phosphate binders once these drugs are included in the bundled payment; and (3) CMS's plans to monitor phosphate binder treatment once these drugs are included in the bundled payment.
GAO reviewed regulations, statutes, and relevant CMS documentation. GAO interviewed CMS officials, five large dialysis organizations that comprised over 85 percent of dialysis facilities in fiscal year 2022, an association representing over 100 small dialysis organizations, and associations representing nephrologists and dialysis patients.
For more information, contact Leslie V. Gordon at (202) 512-7114 or gordonlv@gao.gov.
