Public Health Preparedness: HHS Should Address Strategic National Stockpile Requirements and Inventory Risks
Fast Facts
The U.S. Strategic National Stockpile is a multibillion dollar inventory of medical countermeasures—medication, supplies, and more—that can be used in emergencies. COVID-19 underscored its importance.
The Department of Health and Human Services' inventory planning reports didn't meet most legal requirements enacted in 2019 or communicate risks associated with not meeting recommended inventory levels. This is partly because HHS hasn't updated its processes for completing the reports and a key advisory body was inactive.
Our recommendations address this.
HHS's leadership and coordination of public health emergencies is on our High Risk list.

Highlights
What GAO Found
The Strategic National Stockpile (SNS) is a multibillion dollar inventory of drugs, vaccines, supplies, and other medical countermeasures that can be used in emergencies. GAO reported in August 2022 that to guide inventory purchases from 2015 through 2019, the Department of Health and Human Services (HHS) used a multi-step process involving interagency experts, resulting in annual SNS reviews with inventory recommendations. This process was suspended when the expert group underwent a reorganization, and annual reviews were not completed to inform inventory decisions for fiscal years 2020 through 2022, resulting in purchases based on past reviews and HHS discretion. HHS has since completed reviews to inform inventory decisions for fiscal years 2023 and 2024. However, these reviews did not meet most statutory requirements—such as by including the amount of additional medical countermeasures procured—because HHS did not update its procedures to account for changes enacted in 2019. Until HHS updates its procedures, the agency risks not meeting the statutory requirements designed to give Congress additional information about the SNS inventory.
From fiscal years 2015 through 2021, HHS obligated nearly $5 billion in non COVID-19 appropriations to purchase medical countermeasures, mostly for anthrax and smallpox.
Obligations from Non COVID-19 Appropriations for Strategic National Stockpile from Fiscal Years 2015 through 2021
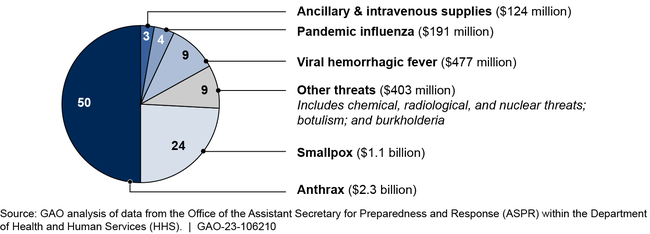
GAO's analysis of SNS reviews shows the SNS contained most medical countermeasure types recommended, but often not in the recommended quantities. HHS officials noted that gaps in quantities are due to budget constraints and acknowledge these gaps present risks. However, the reviews lack key information needed for managing these risks and communicating them to stakeholders, including to Congress. Risk management principles include guidance related to the management and communication of risk. Without an approach for regularly managing risks, HHS and Congress lack assurance the department is most effectively preparing for public health emergencies.
HHS obligated $6.1 billion in COVID-19 relief funds for supplies for the SNS that significantly increased the amount of certain medical countermeasures and expanded the types of countermeasures in the SNS inventory. For example, most of the funding went toward ventilators and personal protective equipment, which resulted in substantial increases in the amount of these medical countermeasures in the SNS relative to what was held prior to the COVID-19 pandemic.
Obligations Using COVID-19 Relief Funds for COVID-19 Supplies Delivered to the Strategic National Stockpile, Fiscal Years 2020 and 2021
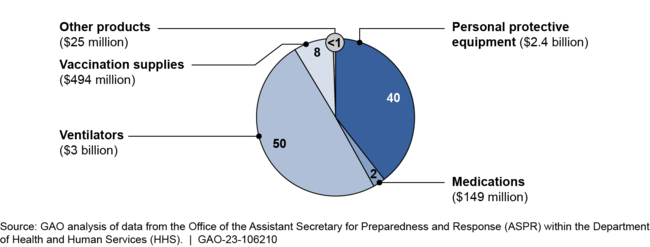
Additionally, the SNS inventory now contains additional finished pharmaceutical products, such as sedatives for use with ventilators. In response to recommendations from HHS, ASPR also took steps to add testing supplies to the SNS inventory in late 2020, including nasal swabs and transfer media. Prior to the COVID-19 pandemic, the SNS did not hold these medical countermeasures.
The COVID-19 response has also been a catalyst for HHS to re-examine SNS operations, including the role, responsibilities, expertise, and inventory needed moving forward. As such, HHS is working to develop a strategic plan to guide future SNS efforts.
Why GAO Did This Study
Recent emergencies have highlighted the importance of preparedness. One key component of the nation's medical response infrastructure is the SNS. The SNS inventory may be deployed to state, local, territorial, tribal, and international governments when needed. GAO placed HHS's leadership and coordination of public health emergencies on its High Risk List in January 2022 (GAO-22-105291) in part due to deficiencies in HHS's management of countermeasures.
The Pandemic and All-Hazards Preparedness and Advancing Innovation Act of 2019 included a provision for GAO to review the SNS. This report examines: (1) the process used to make inventory decisions; (2) non COVID-19 obligations for countermeasures and their alignment with recommendations; and (3) obligations for countermeasures using COVID-19 relief funds, and inventory and operations changes in response to the COVID-19 pandemic.
To conduct this work, GAO reviewed standard operating procedures, statutory requirements, inventory and obligations data, and other documentation; compared HHS actions to risk management practices; and interviewed HHS officials.
This report is a public version of a sensitive report issued in August 2022.
Recommendations
GAO is making three recommendations, including that HHS update procedures for SNS reviews and manage risks associated with inventory gaps. HHS concurred with GAO's recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Office of the Assistant Secretary for Preparedness and Response | The Assistant Secretary for Preparedness and Response should update procedures for how SNS reviews will be conducted in accordance with statutory requirements, including a description of the roles and responsibilities of its interagency partners in the development of the SNS reviews. (Recommendation 1.) |
ASPR concurred with our recommendation and, in June 2024, finalized updated standard operating procedures that are responsive to statutory requirements. Specifically, the procedures define the process that will be used to ensure the SNS reviews (now known as Medical Countermeasures Preparedness Reviews) will be conducted in accordance with statutory requirements, and they outline the roles and responsibilities of interagency partners in their development. As a result of the updated procedures, ASPR will be better positioned to provide Congress additional information about the SNS inventory.
|
| Office of the Assistant Secretary for Preparedness and Response | The Assistant Secretary for Preparedness and Response should develop and document an approach—whether through the standard operating procedures for the SNS reviews or some other mechanism—for ensuring that MCMs under consideration for SNS procurement receive the same consideration regardless of whether they received development funding from BARDA, in accordance with statutory requirements. (Recommendation 2.) |
ASPR concurred with our recommendation and, in June 2024, finalized updated standard operating procedures that are responsive to statutory requirements. Specifically, the procedures clearly state that any SNS procurement recommendations must be the result of equal consideration regardless of the support that guided the products' development. As a result of the documented procedures, ASPR will be positioned to meet its statutory responsibilities related to the SNS inventory. Further, adhering to these procedures will help ensure the SNS contains the most appropriate inventory to be prepared for future public health emergencies.
|
| Office of the Assistant Secretary for Preparedness and Response |
Priority Rec.
The Assistant Secretary for Preparedness and Response should develop and document an approach for regularly managing the risks associated with the gaps between SNS MCM inventory levels and recommended quantities. Such an approach, which could occur as part of the SNS reviews, should clearly prioritize risks, track progress made in addressing the risks, and estimate resources needed to address risks. This approach should involve communicating this information to key decision makers, including Congress. (Recommendation 3.) |
ASPR concurred with our recommendation and, in May 2023 and March 2024, provided updated SNS reviews (now known as Medical Countermeasures Preparedness Reviews) to Congress that better highlight the risks associated with the gaps between SNS MCM inventory levels and recommended quantities. Specifically, these new reviews are responsive to the risk management principles we recommended by prioritizing threats and products, tracking progress of current inventory against recommended quantities, and providing a clear analysis of what resources can be purchased or maintained given current funding levels. They also incorporate required elements added by statute in 2019 which also enhanced ASPR's ability to manage risks. The reviews are delivered to Congress annually, and ASPR officials have provided Congressional staff with additional briefings, information sessions, testimony, and site visits to SNS locations. As a result of incorporating these risk management principles, HHS is better able to manage and communicate SNS risks. Adhering to these principles will help the SNS achieve its mission and provide Congress will key information that can inform decision-making.
|
