Medical Advertising: Federal Oversight of Devices
Fast Facts
There are many TV, print, and online ads for medical devices, such as blood pressure monitors or contact lenses. Direct-to-consumer ads can educate people about devices that may help them. But some of the ads can raise concerns for consumers, doctors, and patients, such as:
False advertising
Ads that create high demand for a product, which can lead to higher costs
Ads that target vulnerable groups
The Food and Drug Administration and the Federal Trade Commission share responsibility for overseeing direct-to-consumer advertising of medical devices. Combined, the agencies took over 300 enforcement actions related to these ads from 2018-2022.

Highlights
What GAO Found
Advertising of medical devices reaches consumers directly through various media, including broadcast, print, the internet, and social media platforms. There are millions of medical devices on the market that can be sold over the counter (e.g., blood pressure monitors), with a prescription (e.g., contact lenses), or in accordance with regulatory restrictions (e.g., cardiac pacemakers). The Food and Drug Administration (FDA) has primary responsibility for overseeing the truth or falsity of advertising for restricted medical devices (e.g., heart valves). These devices can only be sold (1) on oral or written authorization by a licensed practitioner or (2) under conditions specified by regulation in order to assure their safety and effectiveness. The Federal Trade Commission (FTC) has primary responsibility for the truth or falsity of advertising for all devices that are not restricted. FDA and FTC officials stated that the agencies coordinate on an as-needed basis for complaints and concerns.
Food and Drug Administration (FDA) and Federal Trade Commission (FTC) Oversight Responsibilities for Direct-to-Consumer Advertising of Medical Devices
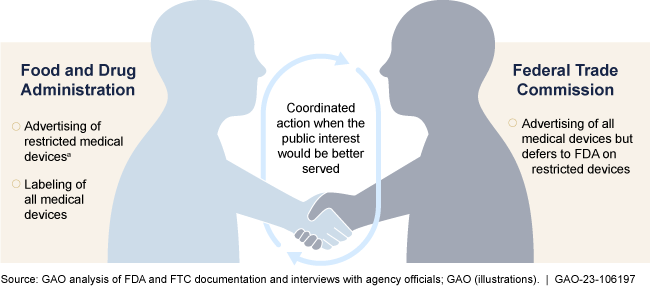
aRestricted medical devices can only be sold (1) when authorized by a licensed practitioner or (2) under conditions specified by regulation.
Between 2018 and 2022, FDA and FTC took 255 and 67 enforcement actions related to medical device advertising issues, respectively, according to agency officials. As a part of their oversight, FDA and FTC also conduct outreach to consumers and businesses to educate them on the rules governing direct-to-consumer advertising.
GAO’s literature search identified no studies that analyzed the effects of direct-to-consumer advertising of medical devices. However, 11 stakeholder groups GAO interviewed identified various effects of, and concerns with, direct-to-consumer advertising of medical devices. For example, five out of 11 groups stated that an advantage of direct-to-consumer advertising of medical devices is that it can provide additional information to consumers, and three groups stated that it can educate consumers. On the other hand, seven of the 11 groups GAO interviewed expressed general concerns associated with direct-to-consumer advertising of medical devices, as follows.
- Information in the advertisements may not be truthful (two groups).
- Advertisements do not contain adequate risk information (one group).
- Advertising can negatively affect the patient-physician relationship by interfering with the ability to arrive at the best treatment option (two groups).
- Advertisements can increase demand for the products, which can increase costs to the patient and the health care system or lead to uses that are not warranted (two groups).
- Target audiences of some direct-to-consumer advertisements may be vulnerable, including those with medical conditions (two groups).
- Consumers can be harmed by the advertised devices (two groups).
Some stakeholder groups also noted future areas of potential concern, including the ease with which consumers can purchase medical products directly from an online advertisement. If the practices involved false or misleading advertising, FDA and FTC officials stated that the agencies would follow their respective procedures for addressing potential violations.
Why GAO Did This Study
The FDA, within the Department of Health and Human Services (HHS), and the FTC have shared responsibility overseeing direct-to-consumer advertising of medical devices in the United States. Medical devices include a broad range of products such as any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use, or software intended to be used in the diagnosis or treatment of disease. Since 2009, GAO has placed FDA’s oversight of medical products, including medical devices, on GAO’s high-risk list.
GAO was asked to review federal oversight and the effects of direct-to-consumer advertising of medical devices. This report describes (1) FDA’s and FTC’s oversight responsibilities for direct-to-consumer advertising of medical devices and (2) identified effects of direct-to-consumer advertising of medical devices. GAO reviewed literature and agency documents and interviewed agency officials and 11 stakeholder groups representing consumers, patients, providers, and manufacturers. GAO selected stakeholder groups by reviewing publically available materials and identifying those that have focused on issues related to direct-to-consumer advertising of medical devices and consumer protection.
For more information, contact Mary Denigan-Macauley at (202) 512-7114 or deniganmacauleym@gao.gov.
