Cancer Clinical Trials: Federal Actions and Selected Non-Federal Practices to Facilitate Diversity of Patients
Fast Facts
Despite more than 3 decades of government policies to improve diversity in clinical cancer trials, certain groups remain underrepresented, including some racial and ethnic groups, women, and low-income individuals. Diversity in clinical trials aims to ensure safe and effective treatments for any patient likely to use them.
To increase diversity in federally-funded clinical cancer trials, agencies broadened eligibility requirements, reimbursed patient out-of-pocket costs to reduce financial burdens, and more. Non-federal cancer centers raised awareness of clinical trials, tailored communication to reach diverse groups of patients, and more.

Highlights
What GAO Found
GAO found that both federal agencies and selected non-federal cancer centers took actions to facilitate participation of patients from diverse backgrounds in cancer clinical trials. Generally, these actions addressed a variety of barriers to participation that are often cited in the literature.
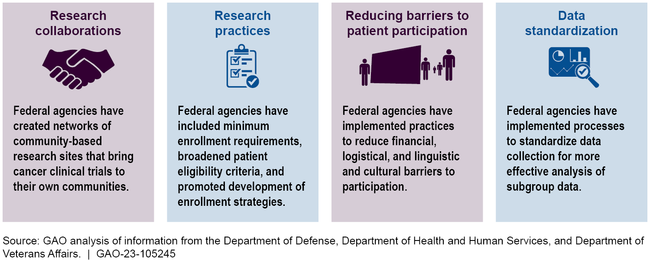
The Department of Health and Human Services, Department of Defense, and Department of Veterans Affairs took actions that have the goal of increasing the proportion of patients from diverse backgrounds enrolled in federally funded cancer clinical trials. These efforts are focused on developing research collaborations, modifying research practices, reducing barriers to patient participation, and collecting and sharing data.
Federal Actions to Facilitate Diversity in Cancer Clinical Trials
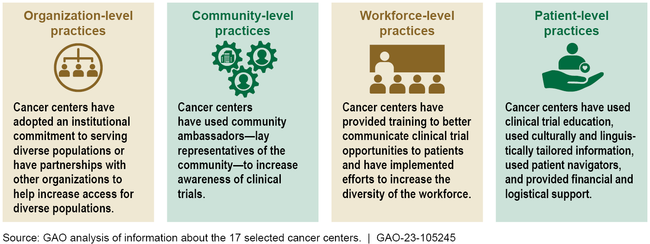
The 17 non-federal cancer centers in GAO's review implemented practices to facilitate the enrollment of patients from diverse backgrounds that were focused on four areas: organization, community, workforce, and patients. Fifteen of the centers implemented practices in at least three of these four areas.
Selected Cancer Center Practices to Facilitate Diversity in Cancer Clinical Trials
Why GAO Did This Study
Diverse representation in clinical trials is important to ensure the safety and efficacy of treatments for the patient population likely to use the treatment being studied. It is also important from an equity perspective as these trials often represent the best available health care. Despite more than 3 decades of government policies intended to improve clinical trial diversity, certain groups remain consistently underrepresented in cancer clinical trials. Those groups include certain racial and ethnic groups, adolescents and young adults, older adults, women, low-income individuals, and individuals from rural communities.
The Henrietta Lacks Enhancing Cancer Research Act of 2019 and House Appropriations Report 116-450 each included a provision that GAO study diverse representation of patients in cancer clinical trials. This report describes (1) actions federal agencies have taken to facilitate enrollment of patients from diverse backgrounds in cancer clinical trials and (2) practices used by selected non-federal cancer centers to facilitate enrollment of patients from diverse backgrounds in cancer clinical trials.
GAO interviewed officials and reviewed documents from federal agencies that fund or conduct cancer clinical trials. GAO also identified 17 cancer centers with a history of enrolling diverse populations in cancer clinical trials and asked them to describe relevant practices that facilitated such enrollment. GAO identified practices facilitating diverse enrollment that were described by or about these centers.
For more information, contact John E. Dicken at (202) 512-7114 or dickenj@gao.gov.
