Drug Safety: COVID-19 Complicates Already Challenged FDA Foreign Inspection Program
Fast Facts
The outbreak of COVID-19 has called greater attention to the United States’ reliance on foreign drug manufacturers. Much of the drug manufacturing for the U.S. market happens overseas—and drugs for treating COVID-19 are no exception.
Food and Drug Administration inspections of foreign and domestic drug manufacturers are critical to ensuring drug safety and effectiveness.
But FDA began to postpone almost all inspections of foreign manufacturing establishments in March 2020 due to COVID-19. We testified that this lack of foreign inspections removes a critical source of information about the quality of drugs manufactured for the U.S. market.
Most Foreign Drug Manufacturing Establishments Shipping to the United States Are in 10 Countries
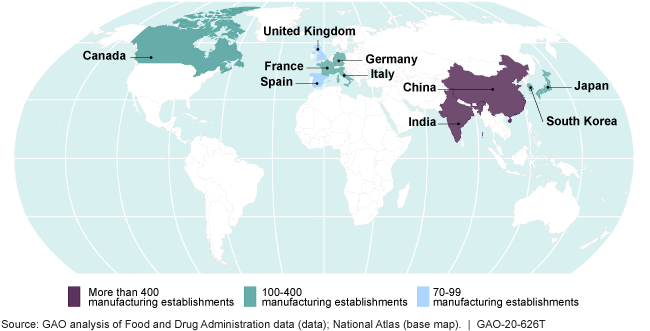
map
Highlights
What GAO Found
In December 2019, GAO found that a growing number of foreign drug manufacturing inspections conducted by the Food and Drug Administration (FDA) were in China and India (43 percent in 2018), where most establishments that manufacture drugs for the United States were located. In fiscal year 2015, FDA, for the first time, conducted more foreign inspections than domestic inspections. However, from fiscal year 2016 through 2018, both foreign and domestic inspections decreased—by about 10 percent and 13 percent, respectively. FDA officials attributed the decline, in part, to vacancies among investigators available to conduct inspections. In March 2020, FDA announced that, due to Coronavirus Disease 2019 (COVID-19), it was postponing almost all inspections of foreign manufacturing establishments. While FDA has indicated it has other tools to ensure the safety of the U.S. drug supply, the lack of foreign inspections removes a critical source of information about the quality of drugs manufactured for the U.S. market.
The 10 Countries with the Most Foreign Drug Establishments Shipping to the United States as of March 2019, by Country
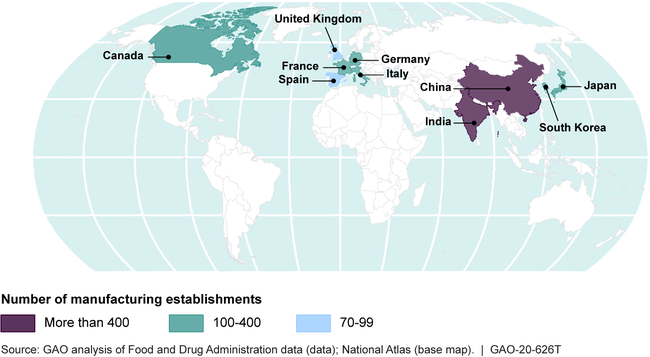
GAO also found that FDA had vacancies among each of the groups of investigators who conduct foreign inspections. FDA had 190 investigators in the United States who conduct the majority of foreign inspections, but an additional 58 positions were vacant. At the time of GAO's December 2019 testimony, FDA was in the process filling 26 of these vacancies, with 32 remaining. However, according to FDA officials, it could be 2 to 3 years before new staff are experienced enough to conduct foreign inspections. FDA also faced persistent vacancies among investigators in its foreign offices.
GAO further found in December 2019 that FDA investigators identified persistent challenges conducting foreign inspections, raising questions about the equivalence of foreign to domestic inspections. Specifically, GAO found:
- While FDA inspections performed in the United States were almost always unannounced, FDA's practice of preannouncing foreign inspections up to 12 weeks in advance may have given manufacturers the opportunity to fix problems ahead of the inspection. Investigators from FDA's China and India offices had conducted some unannounced inspections, but these staff do not perform most of the inspections in these countries (27 percent and 10 percent, respectively).
FDA Estimates of the Amount of Notice Provided to Foreign Drug Establishments Prior to Inspection, Fiscal Year 2018
|
Type of investigator |
Amount of notice provided |
Percentage of inspections involving this investigator type |
|
China office investigator |
0-5 days |
Involved in 27 percent of total number of inspections in China |
|
India office investigator |
0-5 days. |
Involved in 10 percent of total number of inspections in India |
|
U.S.-based investigator |
Generally 12 weeks |
Involved in:
|
Source: Interviews with Food and Drug Administration (FDA) officials and GAO analysis of FDA data. | GAO-20-626T
- FDA was not generally providing translators on foreign inspections. Rather, FDA continued to rely on translators provided by the foreign establishments being inspected, which investigators said raised questions about the accuracy of information FDA investigators collected. For example, one investigator said there was more risk of conflict of interest if the establishment used its own employees to translate. In addition, the establishment representative providing the translation may be someone who does not have the technical language needed, which can make it harder to communicate with establishment staff and facilitate the inspection.
- The overseas travel schedule can present challenges for FDA's domestically based investigators, who conduct the majority of foreign inspections. Domestically based investigators told us there is little flexibility for them to extend foreign inspections during an overseas trip. The inspections they conduct on an overseas trip are scheduled back-to-back in 3-week trips and may involve three different countries. Therefore, extending one inspection would limit the amount of time the investigator has to complete their other scheduled inspections. FDA officials said that inspections conducted by investigators based in China or India (and domestic inspections in the United States) are generally scheduled one at a time and can thus more easily be extended if the investigator needs additional time to pursue potential deficiencies. However, these in-country investigators are not involved in the majority of FDA inspections conducted in China or India.
Why GAO Did This Study
The outbreak of COVID-19 has called greater attention to the United States' reliance on foreign drug manufacturers and further highlighted the importance of ensuring a safe pharmaceutical supply chain. Much of the manufacturing of drugs for treating COVID-19 occurs overseas, which is also true of the majority of other drugs marketed in the United States. While the volume of drugs manufactured overseas for the U.S. market is not fully known, FDA reports that about 70 percent of establishments manufacturing active ingredients and more than 50 percent of establishments manufacturing finished drugs for the U.S. market were located overseas, as of August 2019.
FDA is responsible for overseeing the safety and effectiveness of all drugs marketed in the United States, regardless of where they are produced, and conducts inspections of both foreign and domestic drug manufacturing establishments.
GAO has had long-standing concerns about FDA's ability to oversee the increasingly global pharmaceutical supply chain, an issue highlighted in GAO's High Risk Series since 2009. In particular:
- GAO recommended in 2008 (GAO-08-970) that FDA increase the number of inspections of foreign drug establishments.
- GAO found in 2010 (GAO-10-961) that FDA continued to conduct relatively few foreign inspections than domestic inspections.
- GAO found in 2016 (GAO-17-143) that FDA was conducting more of these foreign drug inspections, and GAO closed its 2008 recommendation to conduct more foreign inspections. However, GAO also reported that FDA may have never inspected many foreign establishments manufacturing drugs for the U.S. market.
In addition, in the summer of 2018, FDA began announcing recalls of blood pressure medications manufactured overseas that were tainted with a potential carcinogen, raising further questions about FDA’s oversight of foreign-manufactured drugs.
This statement is largely based on GAO’s December 2019 testimony (GAO-20-262T) and discusses
1. the number of foreign inspections FDA has conducted,
2. inspection staffing levels, and
3. challenges unique to foreign inspections.
For that testimony, GAO examined FDA data from fiscal years 2012 through 2018 and interviewed investigators from FDA’s 2019 cadre of investigators (who are based in the United States but exclusively conduct foreign drug inspections) and from FDA’s foreign offices in China and India.
For more information, contact Mary Denigan-Macauley at (202) 512-7114 or deniganmacauleym@gao.gov.
