Opioid Use Disorder: Treatment with Injectable and Implantable Buprenorphine
Fast Facts
Treatment for opioid use disorder may involve buprenorphine—a drug that can reduce or eliminate withdrawal symptoms and prevent relapse.
We reviewed the use of long-acting injectable and implantable forms of buprenorphine for opioid use disorder. These forms release the drug over 1- or 6-month periods respectively.
Providers issued about 7,250 prescriptions for injectable or implantable buprenorphine in FY 2019.
Providers and pharmacy representatives said there is a low risk of these forms of the drug being diverted to the illegal marketplace.
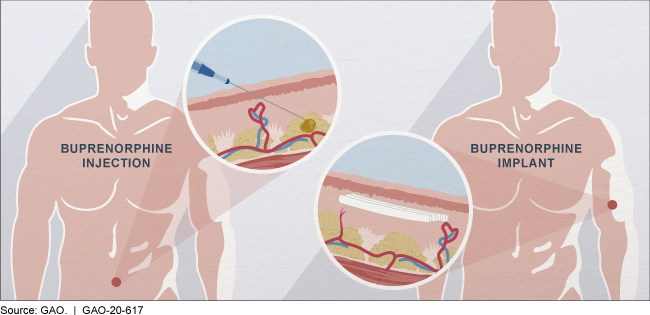
Buprenorphine may be injected into the abdomen or implanted in the upper arm.
Highlights
What GAO Found
Of the medications used to treat opioid use disorder (OUD), only buprenorphine is both a controlled substance and available as an injection or implant. Buprenorphine is used to treat patients with OUD because it reduces or eliminates opioid withdrawal symptoms and blunts the euphoria or dangerous side effects of other opioids, such as heroin. When used to treat OUD, buprenorphine, in any form, is subject to additional laws and regulations that are overseen by the Drug Enforcement Administration (DEA), within the Department of Justice (DOJ) and the Substance Abuse and Mental Health Services Administration (SAMHSA), within the Department of Health and Human Services (HHS). To ensure patient safety when injectable and implantable buprenorphine is used, the Food and Drug Administration (FDA), within HHS has also required drug companies to establish risk evaluation and mitigation strategies to help ensure the benefits of these medications outweigh their risks.
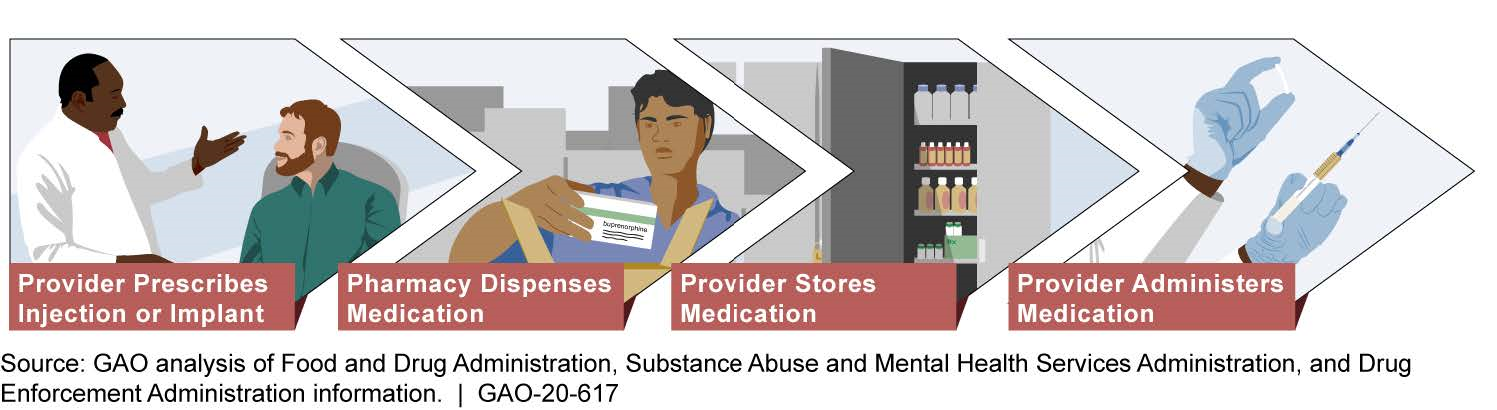
Providers and pharmacies must follow a number of specific steps based on federal requirements when providing treatment with injectable and implantable buprenorphine. Providers are responsible for prescribing, storing, and administering injectable and implantable buprenorphine, while pharmacies are responsible for dispensing these medications (see figure). Representatives GAO interviewed from provider groups and pharmacies said they did not find the steps involved in treating patients to be difficult overall. However, they stated that careful and timely coordination with each other and patients is needed at key steps of the process to ensure that the patient receives treatment. Representatives from provider groups and pharmacies reported that the risk of diversion of injectable and implantable buprenorphine is low. For example, all of the provider groups GAO spoke with said that diversion of injectable or implantable buprenorphine is unlikely, and representatives from three of the six provider groups said that the design of these formulations reduces opportunities for diversion due to how they are administered.
Process for Treating Opioid Use Disorder with Injectable and Implantable Buprenorphine
The use of injectable and implantable buprenorphine to treat OUD is relatively low compared to oral forms of buprenorphine. HHS has reported that about 7,250 prescriptions were issued for injectable and implantable buprenorphine in fiscal year 2019, compared to over 700,000 patients who received buprenorphine prescriptions for oral formulations to treat OUD or pain in that year.
Why GAO Did This Study
In 2018, SAMHSA estimated that about one-quarter of the estimated 2 million people with OUD had received some form of substance use treatment in the prior year. One form of treatment—medication-assisted treatment (MAT)— combines behavioral therapy with the use of certain medications. HHS has identified expanding access to treatment for OUD as an important strategy for reducing opioid morbidity and mortality, which includes increasing the number of injectable and implantable buprenorphine prescriptions.
Congress included a provision in the SUPPORT Act for GAO to review access to and the potential for the diversion of controlled substances administered by injection or implantation. This report focuses on injectable and implantable controlled substances that can be used to treat OUD and specifically, describes the process for treating OUD with injectable and implantable buprenorphine and what is known about their use.
GAO reviewed laws, regulations, and documentation from DEA, FDA, and SAMHSA governing the process of providing treatment with buprenorphine and interviewed officials from those agencies. GAO also interviewed representatives from stakeholder groups representing MAT providers; drug companies that manufacture injectable or implantable buprenorphine; and pharmacies that dispense these medications. HHS and DOJ reviewed a draft of this report, and GAO incorporated their technical comments, as appropriate.
For more information, contact James Cosgrove at (202) 512-7114 or cosgrovej@gao.gov.
