Medicaid Demonstrations: Approvals of Major Changes Need Increased Transparency
Fast Facts
About a third of Medicaid spending is for demonstrations, which allow states to test new approaches to delivering services. States and the federal government are supposed to be transparent about the demonstrations that are proposed and give the public a chance to weigh in. Is that happening?
The short answer is sometimes. Transparency has improved, but there are still significant gaps. For example, the federal government doesn't always require states to share the projected effects of proposals, even when they could significantly affect beneficiary eligibility.
We recommended ways for Medicaid to address these issues.
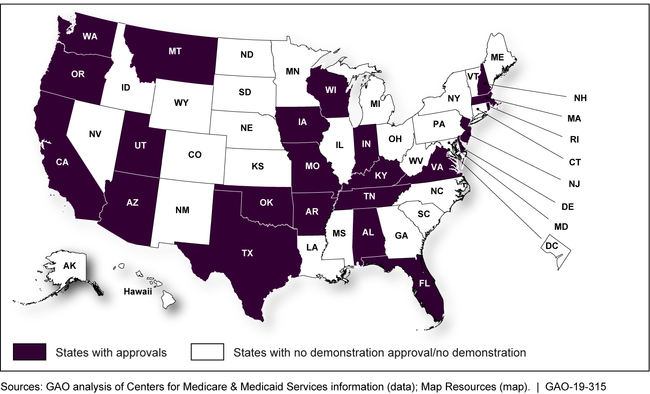
States that received a demonstration approval from January 2017 through May 2018
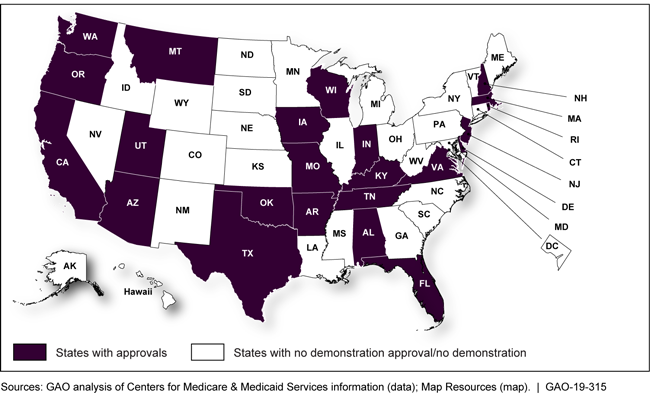
Map showing states that received demonstration approvals from January 2017 through May 2018
Highlights
What GAO Found
Medicaid demonstrations allow states flexibility to test new approaches for providing coverage and delivering Medicaid services. Since 2012, the Centers for Medicare & Medicaid Services (CMS), which oversees demonstrations, has developed procedures to improve the transparency of the approval process. For example, CMS reviews demonstration applications (including for new demonstrations, extensions, and amendments to existing demonstrations) for their compliance with applicable transparency requirements, including that states seek public input on their applications.
States that Received Demonstration Approvals, January 2017–May 2018
However, GAO found weaknesses in CMS's policies for ensuring transparency.
Changes to pending applications for new demonstrations or extensions. CMS lacks policies for ensuring transparency when states submit major changes to pending applications. For two of the four approvals of new demonstrations or extensions GAO reviewed in-depth, states submitted changes to their applications that could have significant effects on beneficiaries (such as disenrollment or other penalties) without first obtaining public comment on these changes at the state level.
Amendments to existing demonstrations. CMS's transparency requirements for amendments are limited. For example, CMS does not require amendment applications to include how the changes may affect beneficiary enrollment or report on concerns raised in state public comments. However, states have proposed major changes—such as work and community engagement requirements—through amendments, raising concerns that major changes to states' demonstrations are being approved without a complete understanding of their impact.
Why GAO Did This Study
Section 1115 demonstrations are a significant component of Medicaid spending and affect the care of millions of beneficiaries. The Patient Protection and Affordable Care Act required the Department of Health and Human Services (HHS) to establish procedures to ensure transparency in approvals of new demonstrations and extensions to existing demonstrations. The act did not address amendments, which are subject to long-standing guidance on public input.
GAO was asked to examine the transparency of demonstration approvals. Among other things, this report examines CMS's transparency policies and procedures for new demonstrations and extensions, and amendments to existing demonstrations. To review a variety of approval types across a large number of states, GAO examined all approvals of new demonstrations and extensions of and amendments to existing demonstrations granted from January 2017 through May 2018. GAO also conducted in-depth reviews of one approval in each of seven states, selected to include at least two approvals of each type. GAO reviewed demonstration documentation for these states, and interviewed state and federal Medicaid officials. GAO also assessed CMS's procedures against federal internal control standards.
Recommendations
CMS should develop policies for ensuring transparency when states (1) submit major changes to pending demonstration applications and (2) propose amendments to existing demonstrations. HHS concurred with these recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Centers for Medicare & Medicaid Services | The Administrator of CMS should develop and communicate a policy that defines when changes to a pending section 1115 demonstration application are considered major and should prompt a new review of the application against the transparency requirements applicable to the pending application. (Recommendation 1) |
In response to this recommendation, HHS stated that existing regulations permit CMS at its discretion to direct an additional public comment period when states make a modification to an application that substantially changes the design. In January 2026, HHS officials said they continue to consider this recommendation, including exploring the timing and means for defining "substantial change" and an approach for determining whether and when additional public comment periods are needed. We will continue to monitor CMS's actions in this area.
|
| Centers for Medicare & Medicaid Services |
Priority Rec.
The Administrator of CMS should develop and communicate a policy whereby applications for section 1115 demonstration amendments that may have significant impact are subject to transparency requirements comparable to those for new demonstrations and extensions. (Recommendation 2) |
In response to this recommendation, HHS stated that it plans to implement a policy applying state public input processes and application criteria to amendments proposing significant or substantial changes in the same manner as for new demonstrations. In January 2026, CMS officials said the agency still plans to develop criteria for what constitutes a substantial change in a demonstration, for both amendments as well as extensions to demonstrations. CMS, however, did not have a timeline for when such criteria would be developed. We will continue to monitor CMS's actions in this area and will close this recommendation once this policy guidance is issued.
|
