Drug Discount Program: Update on Agency Efforts to Improve 340B Program Oversight
Fast Facts
Under the 340B Drug Pricing Program, if drug manufacturers want Medicaid to cover their drugs, they are required to sell outpatient drugs at discounted prices to covered entities—such as eligible clinics and hospitals.
Our 2011 report found weaknesses in the Health Resources & Services Administration's (HRSA) oversight of the 340B program.
This testimony describes HRSA’s progress in addressing our recommendations: it now audits entities to ensure they comply with program rules, and clarified its guidance on equitable distribution of drugs to 340B entities. Two other recommendations on clarifying eligibility guidance have not been addressed.
Growth in Covered Entity Sites Participating in the 340B Program, 2013-2017
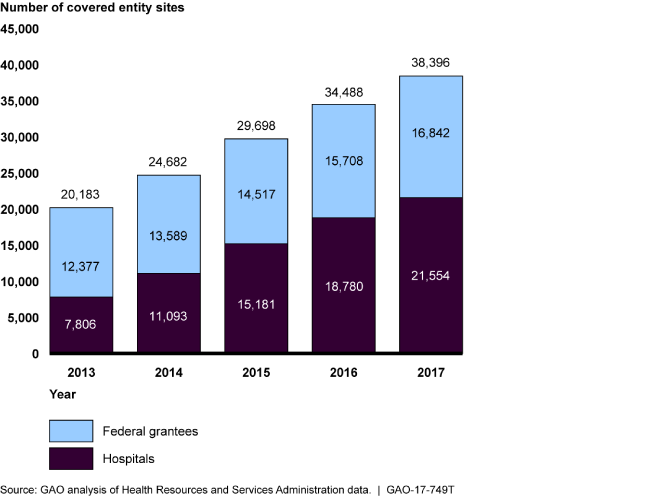
Bar chart showing that the number of sites participating in the program has almost doubled over 5 years.
Highlights
What GAO Found
The 340B Drug Pricing Program requires drug manufacturers to sell outpatient drugs at discounted prices to covered entities—eligible clinics, hospitals, and others—to have their drugs covered by Medicaid. Covered entities are only allowed to provide 340B drugs to certain eligible patients. Entities dispense 340B drugs through in-house pharmacies or contract pharmacies, which are outside pharmacies entities contract with to dispense drugs on their behalf. The number of contract pharmacies has increased significantly in recent years.
In its September 2011 report, GAO found that the Health Resources and Services Administration's (HRSA) oversight of the 340B program was inadequate to ensure compliance with program rules, and GAO recommended actions that HRSA should take to improve program integrity, particularly given significant growth in the program in recent years. HRSA has taken steps to address two of GAO's four recommendations:
- HRSA initiated audits of covered entities . GAO found that HRSA's oversight of the 340B Program was weak because it primarily relied on covered entities and manufacturers to ensure their own compliance with program requirements and HRSA engaged in few oversight activities. GAO recommended that HRSA conduct audits of covered entities and in fiscal year 2012, HRSA implemented a systematic approach to conducting annual audits of covered entities. HRSA now conducts 200 audits a year, which have identified instances of non-compliance with program requirements, including the dispensing of drugs to ineligible patients.
- HRSA clarified guidance for manufacturers. GAO found a lack of specificity in guidance for manufacturers for handling cases in which distribution of drugs is restricted, such as when there is a shortage in drug supply. GAO recommended that HRSA refine its guidance. In May 2012, HRSA clarified its policy for when manufacturers restricted distribution of a drug and provided additional detail on the type of information manufacturers should include in their restricted distribution plans.
- HRSA has not clarified guidance on two issues. GAO also found that HRSA guidance on (1) the definition of an eligible patient and (2) hospital eligibility criteria for program participation lacked specificity and recommended that HRSA clarify its guidance. HRSA agreed that clearer guidance was necessary and, in 2015, released proposed guidance that addressed both issues. However, earlier this year, the agency withdrew that guidance in accordance with recent directives to freeze, withdraw, or postpone pending federal guidance.
Given particular concerns that the significant escalation in the number of contract pharmacies poses a potential risk to the integrity of the 340B Program, GAO was asked to examine this issue and expects to issue a future report, in which it plans to address the extent to which covered entities use contract pharmacies; financial arrangements between covered entities and pharmacies; the provision of discounts on drugs dispensed by contract pharmacies to low-income, uninsured patients; and how covered entities and HRSA ensure compliance with 340B program requirements at contract pharmacies.
Why GAO Did This Study
According to HRSA, the purpose of the 340B Program, which was created in 1992, is to enable covered entities to stretch scarce federal resources to reach more eligible patients, and provide more comprehensive services. Covered entities can provide 340B drugs to patients regardless of income or insurance status and generate revenue by receiving reimbursement from patients insurance. The program does not specify how this revenue is to be used or whether discounts are to be passed on to patients. The number of participating covered entity sites—currently about 38,000—has almost doubled in the past 5 years and the number of contract pharmacies increased from about 1,300 in 2010 to around 18,700 in 2017. In recent years, questions have been raised regarding oversight of the 340B Program, particularly given the program's growth over time.
In September 2011, GAO identified inadequacies in HRSA's oversight of the 340B program and made recommendations for improvement. This statement describes (1) HRSA actions in response to GAO recommendations to improve its program oversight, and (2) ongoing GAO work regarding the 340B program and HRSA oversight.
For this statement, GAO obtained information and documentation from HRSA officials about any significant program updates and steps they have taken to implement the 2011 GAO recommendations. More detailed information on the objectives, scope, and methodology can be found in GAO's September 2011 report.
For more information, contact Debra A. Draper at (202) 512-7114 or draperd@gao.gov.
