High-Containment Laboratories: Improved Oversight of Dangerous Pathogens Needed to Mitigate Risk
Highlights
What GAO Found
The total number of incidents involving incomplete inactivation—a process to destroy the hazardous effects of pathogens while retaining characteristics for future use—that occurred from 2003 through 2015 is unknown for several reasons. One key reason is that the Select Agent Program—operated by the Departments of Health and Human Services (HHS) and Agriculture (USDA) to oversee certain dangerous pathogens, known as select agents—does not require laboratories to identify such incidents on reporting forms. According to the program, 10 incidents occurred from 2003 through 2015. However, GAO identified an additional 11 incidents that the program did not initially identify. Because the program cannot easily identify incidents involving incomplete inactivation, it does not know the frequency or reason they occur, making it difficult to develop guidance to help mitigate future incidents. The 21 identified incidents involved a variety of pathogens and laboratories, as shown below.
Figure: Twenty-one Identified Incidents Involving Incomplete Inactivation that Occurred from 2003 through 2015 by Pathogen and Laboratory Type
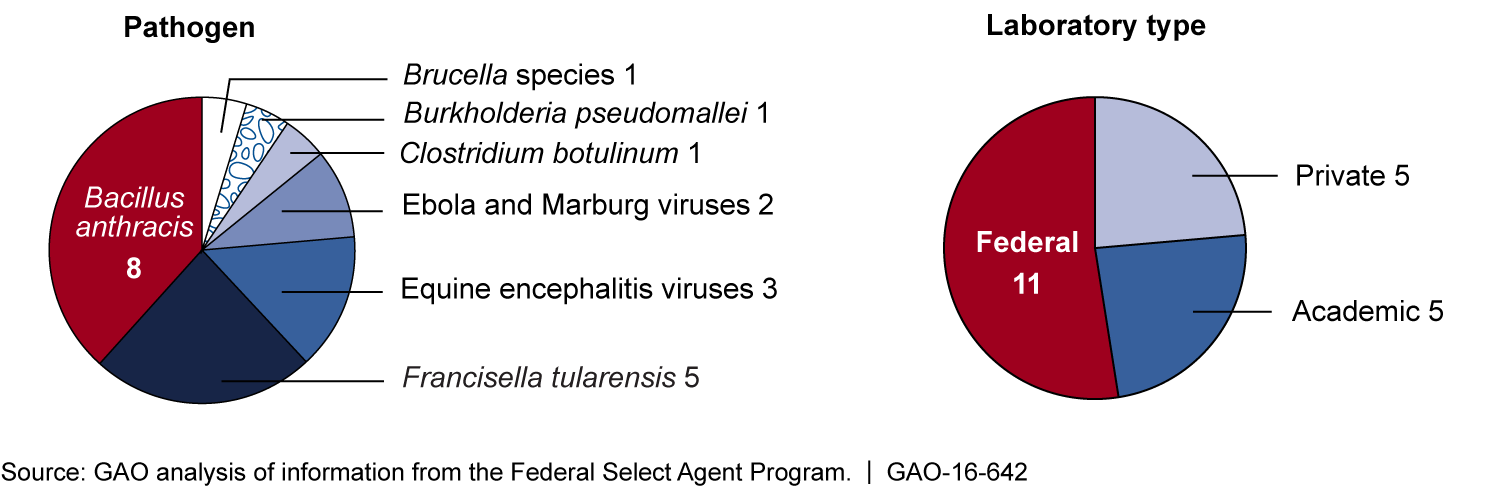
Several challenges affect the implementation of inactivation in high-containment laboratories, including gaps in scientific knowledge and limited guidance. For example, there is limited federal guidance for researchers on the development and validation of inactivation protocols. Validation helps ensure protocols are scientifically sound and produce consistent results. Due to limited guidance, laboratories varied in their interpretation of validated methods of inactivation, resulting in researchers applying differing levels of rigor. Without more comprehensive guidance, as called for by experts, protocols will vary in their scientific soundness, increasing the risk of incomplete inactivation.
The Select Agent Program did not consistently refer incidents involving incomplete inactivation for further investigation and enforcement for violations of select agent regulations. For example, the program referred incidents involving incomplete inactivation at various laboratories, but did not refer two incidents in 2014 that occurred at HHS. A memorandum of understanding between HHS and USDA states that the program should handle incidents consistently. GAO found, however, that the program does not have a consistent, written set of criteria for handling incidents. Without such criteria, the program risks inconsistent enforcement of select agent regulations. This further highlights GAO’s previous finding that existing federal oversight of high-containment laboratories is fragmented and self-policing.
Why GAO Did This Study
Several incidents involving the shipment of live pathogens, thought to be inactivated, have recently occurred, potentially exposing people to dangerous pathogens that cause infectious diseases, such as the bacterium that causes anthrax.
GAO was asked to evaluate issues related to inactivation of pathogens in high-containment laboratories. This report examines (1) the extent to which incidents involving incomplete inactivation occurred from 2003 through 2015, (2) any challenges that may affect the implementation of inactivation in high-containment laboratories, and (3) the extent to which the Select Agent Program referred violations and enforced regulations related to incidents involving incomplete inactivation. GAO convened an expert meeting with the assistance of the National Academy of Sciences to discuss various issues surrounding inactivation. GAO also reviewed relevant laws, regulations, and guidance, and interviewed officials at laboratories that conduct inactivation.
Recommendations
GAO is making six recommendations to HHS and USDA to, among other things, improve the Select Agent Program’s oversight of inactivation by revising reporting forms, improving guidance for development and validation of inactivation protocols, and developing consistent criteria for enforcement of incidents involving incomplete inactivation. HHS and USDA agreed with GAO’s recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Department of Agriculture | To understand the extent to which incomplete inactivation occurs and whether incidents are being properly identified, analyzed, and addressed, the Secretary of Agriculture should direct the Animal and Plant Health Inspection Service (APHIS) to develop clear definitions of inactivation that are consistent across the Select Agent Program. |
In January and March 2017, the U.S. Department of Agriculture, in collaboration with the Department of Health and Human Services, issued updated select agent regulations and guidance that included clear definitions of inactivation and a validated inactivation procedure that are consistent across the Federal Select Agent Program.
|
| Department of Health and Human Services | To understand the extent to which incomplete inactivation occurs and whether incidents are being properly identified, analyzed, and addressed, the Secretary of Health and Human Services should direct the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH) to develop clear definitions of inactivation for use within their respective guidance documents that are consistent across the Select Agent Program, NIH's oversight of recombinant pathogens, and the Biosafety in Microbiological and Biomedical Laboratories manual. |
As of November 2020, the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH) have developed clear definitions of inactivation that are consistent across the Select Agent Program, NIH's oversight of recombinant pathogens, and the Biosafety in Microbiological and Biomedical Laboratories manual. In November 2020, CDC and NIH released the 6th edition of the Biosafety in Microbiological and Biomedical Laboratories manual, which includes a definition of inactivation that is consistent with definitions of inactivation in previously-released guidance documents for the Select Agent Program and NIH's oversight of recombinant pathogens. This fulfills the intent of the recommendation.
|
| Department of Agriculture | To understand the extent to which incomplete inactivation occurs and whether incidents are being properly identified, analyzed, and addressed, the Secretary of Agriculture should direct APHIS to revise reporting forms to help identify when incidents involving incomplete inactivation occur and analyze the information reported to help identify the causes of incomplete inactivation to mitigate the risk of future incidents. |
In November 2017, the Federal Select Agent Program updated its reporting form to include "inactivation failure" as a type of incident. In addition, in May 2018, the Federal Select Agent Program approved a document that describes procedures for reviewing and analyzing reported incidents.
|
| Department of Health and Human Services | To understand the extent to which incomplete inactivation occurs and whether incidents are being properly identified, analyzed, and addressed, the Secretary of Health and Human Services should direct CDC and NIH to revise reporting forms within their respective areas of oversight to help identify when incidents involving incomplete inactivation occur and analyze the information reported to help identify the causes of incomplete inactivation to mitigate the risk of future incidents. |
In August 2016, the National Institutes of Health (NIH) revised its template for reporting incidents subject to the NIH Guidelines to include a specific checkbox for entities to identify incidents that involve incomplete inactivation. In November 2017, the Federal Select Agent Program updated its reporting form to include "inactivation failure" as a type of incident. In addition, in May 2018, the Federal Select Agent Program approved a document that describes procedures for reviewing and analyzing reported incidents.
|
| Department of Agriculture | To increase scientific information on inactivation and viability testing, the Secretaries of Health and Human Services and Agriculture should coordinate research efforts and take actions to help close gaps in the science of inactivation and viability testing across high-containment laboratories. |
In September 2020, United States Department of Agriculture (USDA) officials stated that the department, in coordination with the Department of Health and Human Services (HHS), had taken actions to help close gaps in the science of inactivation and viability testing across high-containment laboratories. In particular, USDA is co-chairing the Applied Biosafety Research Working Group, which was established by the National Science and Technology Council's Subcommittee on Biological Defense Research and Development (recently renamed Health Security Threats) and which HHS is also a member of. This working group developed a draft road map that identified five broad areas with research gaps, according to a USDA document and officials. One of the areas includes identification of inactivation, decontamination, and sterilization procedures. The draft road map draws heavily from a 2019 applied biosafety research workshop, which engaged federal agencies and biorisk experts to identify knowledge gaps, according to USDA officials. Officials said that USDA has also taken other actions to address this recommendation, including conducting additional applied biosafety research.
|
| Department of Health and Human Services | To increase scientific information on inactivation and viability testing, the Secretaries of Health and Human Services and Agriculture should coordinate research efforts and take actions to help close gaps in the science of inactivation and viability testing across high-containment laboratories. |
In September 2020, Department of Health and Human Services (HHS) officials stated that the department, in coordination with the United States Department of Agriculture (USDA), had taken actions to help close gaps in the science of inactivation and viability testing across high-containment laboratories. In particular, HHS and USDA are members of the Applied Biosafety Research Working Group, which was established by the National Science and Technology Council's Subcommittee on Biological Defense Research and Development (recently renamed Health Security Threats). This working group developed a draft road map that identified five broad areas with research gaps, with one of the areas including identification of inactivation, decontamination, and sterilization procedures, according to HHS and USDA officials. The draft road map draws heavily from a 2019 applied biosafety research workshop, which engaged federal agencies and biorisk experts to identify knowledge gaps, according to HHS and USDA officials. In addition, HHS has taken other actions to address this recommendation, including holding a symposium in May 2017 to share experiences on the use of various inactivation methods and successes and failures and discuss gaps in scientific information.
|
| Department of Agriculture | To help ensure that inactivation protocols are scientifically sound and are effectively implemented, the Secretary of Agriculture should direct APHIS to create comprehensive and consistent guidance for the development, validation, and implementation of inactivation protocols--to include the application of safeguards--across the Select Agent Program. |
In March 2017, the United States Department of Agriculture (USDA), in collaboration with the Department of Health and Human Services (HHS), issued Federal Select Agent Program guidance on the inactivation of select agents and toxins. According to USDA, this guidance is intended to assist entities with development and implementation of inactivation procedures and viability testing. Our review found the guidance to generally be comprehensive, consistent, and applicable across the Federal Select Agent Program. This fulfills the intent of the recommendation.
|
| Department of Health and Human Services | To help ensure that inactivation protocols are scientifically sound and are effectively implemented, the Secretary of Health and Human Services should direct CDC and NIH to create comprehensive and consistent guidance for the development, validation, and implementation of inactivation protocols--to include the application of safeguards--across the Select Agent Program, NIH's oversight of recombinant pathogens, and the Biosafety in Microbiological and Biomedical Laboratories manual. |
As of November 2020, the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH) have developed comprehensive guidance on inactivation that is consistent across the Select Agent Program, NIH's oversight of recombinant pathogens, and the Biosafety in Microbiological and Biomedical Laboratories manual. In November 2020, CDC and NIH released the 6th edition of the Biosafety in Microbiological and Biomedical Laboratories manual, which includes a new appendix on inactivation that is consistent with previously-released guidance for the Select Agent Program and NIH's oversight of recombinant pathogens. This appendix includes comprehensive information on the development, validation, and implementation of inactivation protocols.
|
| Department of Health and Human Services | To help ensure that dangerous pathogens can be located in the event there is an incident involving incomplete inactivation, the Secretary of Health and Human Services should direct the Directors of CDC and NIH, when updating the Biosafety in Microbiological and Biomedical Laboratories manual, to include guidance on documenting the shipment of inactivated material. |
In November 2020, the Centers for Disease Control and Prevention and National Institutes of Health released the 6th edition of the Biosafety in Microbiological and Biomedical Laboratories manual, which includes a new appendix on inactivation. This appendix includes a section on the tracking of and communication about inactivated samples, including guidance on recordkeeping and the shipment of inactivated material. This fulfills the intent of the recommendation.
|
| Department of Agriculture | To help ensure more consistent enforcement for violations involving incomplete inactivation of select agents, the Secretary of Agriculture should direct APHIS to develop and implement consistent criteria and documentation requirements for referring violations to investigative entities and enforcing regulations related to incidents involving incomplete inactivation. |
APHIS, in coordination with CDC, has taken several joint steps to help ensure consistency in the Federal Select Agent Program's enforcement for violations involving incomplete inactivation of select agents. First, in September 2017, the Federal Select Agent Program finalized a document that provides criteria on when to refer violations and options for enforcement. The document categorizes regulatory departures, grouped by level of risk, along a spectrum of severity with associated enforcement options. In addition, in the spring of 2018, the Federal Select Agent Program finalized three joint standard operating procedures related to enforcement of the select agent regulations. The first one, finalized in March 2018, outlines procedures, including documentation requirements, for instituting compliance actions against entities determined to be in violation of the select agent regulations. The second one, finalized in April 2018, describes procedures for denying entity applications or suspending or revoking entity registrations to possess, use, and transfer select agents. The third one, finalized in May 2018, describes procedures for instituting corrective action plans for entities in violation of the select agent regulations.
|
| Department of Health and Human Services | To help ensure more consistent enforcement for violations involving incomplete inactivation of select agents, the Secretary of Health and Human Services should direct CDC to develop and implement consistent criteria and documentation requirements for referring violations to investigative entities and enforcing regulations related to incidents involving incomplete inactivation. |
CDC, in coordination with APHIS, has taken several joint steps to help ensure consistency in the Federal Select Agent Program's enforcement for violations involving incomplete inactivation of select agents. First, in September 2017, the Federal Select Agent Program finalized a document that provides criteria on when to refer violations and options for enforcement. The document categorizes regulatory departures, grouped by level of risk, along a spectrum of severity with associated enforcement options. In addition, in the spring of 2018, the Federal Select Agent Program finalized three joint standard operating procedures related to enforcement of the select agent regulations. The first one, finalized in March 2018, outlines procedures, including documentation requirements, for instituting compliance actions against entities determined to be in violation of the select agent regulations. The second one, finalized in April 2018, describes procedures for denying entity applications or suspending or revoking entity registrations to possess, use, and transfer select agents. The third one, finalized in May 2018, describes procedures for instituting corrective action plans for entities in violation of the select agent regulations.
|
