Science & Tech Spotlight: COVID-19 Vaccine Development [Updated on June 29, 2021]
Fast Facts
[This is an update to the previous COVID-19 vaccine development spotlight. It was updated to include new and updated information on the federal effort to support the development, manufacture, and delivery of COVID-19 vaccines. The PDF for the previous spotlight is available here.]
Vaccines can help save lives and speed economic recovery. U.S.-funded efforts to develop a COVID-19 vaccine have led to multiple vaccines available to the public in record time.
However, challenges to vaccine development and production remain. For instance, mutations in coronaviruses may require developing new or modified vaccines. Additionally, there are shortages of manufacturing facilities and personnel with specialized skills to run these facilities.

Highlights
Why This Matters
SARS-CoV-2, which causes COVID-19, has killed millions worldwide. Vaccines can help save lives and speed economic recovery. Developing a safe, effective vaccine is a complicated, costly, and typically lengthy process that has historically had a low success rate. U.S.-funded efforts have made several vaccines available to the public in record time.
The Technology
What is it? Vaccines protect people from disease by triggering the immune system to produce antibodies that will fight the pathogen attacking the body. In the case of COVID-19, the pathogen is the virus SARS-CoV-2. Developing a vaccine is an expensive and typically lengthy process because it involves a rigorous series of steps to first identify a potential vaccine “candidate,” then assess it for safety and effectiveness. Billions of vaccine doses designed to fight COVID-19 have already been administered as of June 2021 across the U.S. and the world, and death rates in some countries have dropped dramatically.
How does it work? A vaccine can use a pathogen that has been modified to be safe or a molecule that resembles a part of the pathogen, triggering the immune system to produce antibodies. If the vaccinated person is exposed later to the pathogen, their body will produce those antibodies again, increasing their chances of fighting off infection.
Development starts with identifying a pathogen “target,” such as a protein, that can induce an immune reaction. Researchers create a vaccine candidate similar to that target that will induce production of antibodies effective against the pathogen. The vaccine candidate moves through stages of development, assessment, authorization, and licensure (fig. 1).
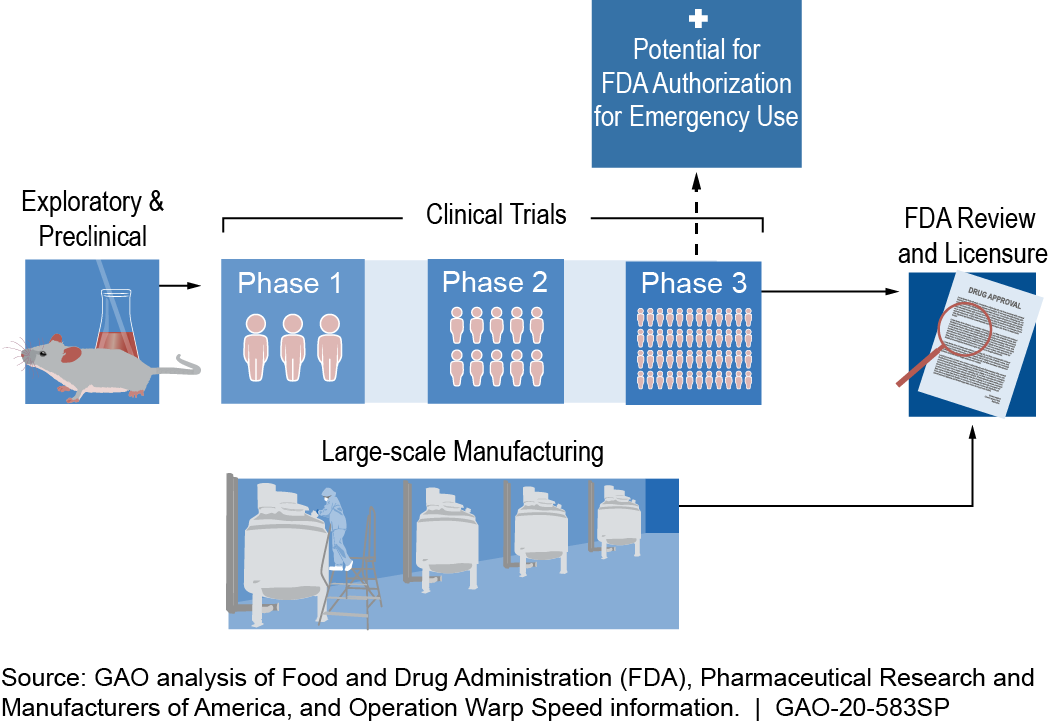
Figure 1. Example of a COVID-19 vaccine timeline. Specific steps and timelines may vary. According to FDA, any COVID-19 vaccine that initially receives an emergency use authorization is expected to be reviewed and licensed through a biologics license application.
Under normal circumstances, the entire process typically takes 10 to 15 years, with more than 65 percent of candidates failing, according to an MIT study. There has been an effort to expedite this process for COVID-19 vaccines. More than 250 COVID-19 vaccines have been in development globally. Of those, the federal government awarded billions of dollars to companies for the development, manufacture, or distribution of six vaccine candidates under a partnership between the Departments of Defense (DOD), and Health and Human Services (HHS). These six candidates use three different “platforms,” or mechanisms to prompt the body to produce antibodies (fig. 2). The three platforms generate proteins that mimic part of the spike protein found on the surface of SARS-CoV-2. The spike protein alone does not cause a COVID-19 infection but may be sufficient to produce an immune response.
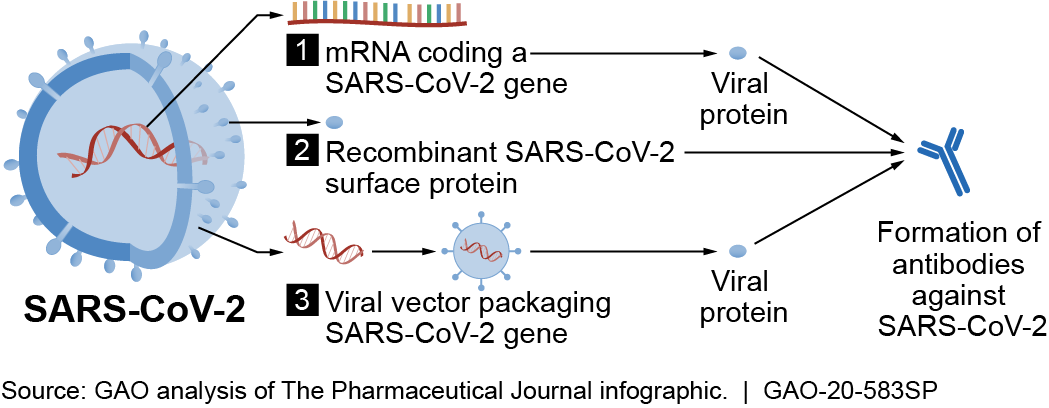
Figure 2. COVID-19 vaccine candidates use different mechanisms, such as those shown above, to prompt the body to produce antibodies against SARS-CoV-2.
The first platform uses a molecule called mRNA, which is specifically coded to generate proteins that induce an immune response. This is a newer method of vaccine development.
The second platform uses recombinant proteins, which are produced by genetically engineered bacteria or other cells, to induce an immune response. This platform is already being used successfully against other viruses, such as the human papillomavirus (HPV), which can cause cervical cancer.
The third platform uses another virus—called adenovirus—removed of its infectious aspects, making it safe as a “vector” to deliver a piece of a pathogen to produce proteins that induce an immune response. This platform is being used in U.S. clinical trials for HIV and Ebola vaccine candidates.
How mature is it? The process for developing a new vaccine as outlined by the Food and Drug Administration (FDA) is well established. In the exploratory stage, the pathogen target and candidate vaccine are identified. In the preclinical stage, researchers use cells and animals to assess safety and produce evidence of clinical promise, evaluated by the candidate’s ability to elicit a protective immune response.
During clinical trials, the number of people participating is increased at each successive phase. Safety, efficacy, proposed doses, schedule of immunizations, and method of delivery are evaluated. Clinical trials can be terminated for various reasons, including observations of side effects considered to be serious adverse events. For some COVID-19 vaccine candidates, large-scale manufacturing began during clinical trials so that doses could be available for distribution upon emergency use authorization (EUA) of the vaccine.
FDA authorizes and licenses vaccines to be marketed in the U.S. Following a declared emergency, FDA can issue EUAs to allow temporary use of vaccine candidates that have not yet been licensed, provided certain statutory criteria are met. For example, it must be reasonable to believe that the vaccine may be effective and that the known and potential benefits of the vaccine outweigh the known and potential risks. FDA’s role includes oversight of manufacturing and continual monitoring after authorization or licensure.
FDA has four programs to facilitate and expedite the development and review of new vaccine candidates for the prevention of serious or life-threatening conditions such as COVID-19. These programs can help expedite some processes, such as a rolling review of portions of applications, to bring vaccines to market more quickly. Vaccine developers could use one or more of these programs.
Opportunities
Several technologies and initiatives offer opportunities to accelerate vaccine development. For example:
- Genomic tools. Tools that provide information about a pathogen’s genetic makeup can make it easier for researchers to quickly select a target that can trigger an immune response.
- Collaboration and partnerships. DOD and HHS are working with companies and manufacturing partners to help accelerate the development, manufacture, and distribution of COVID-19 vaccines.
- Multiple vaccine mechanisms. Simultaneous testing of multiple vaccine mechanisms can improve the chances of developing a successful vaccine faster.
Challenges
- Virus mutations. Coronaviruses, such as SARS-CoV-2, can mutate and potentially reduce or eliminate a vaccine’s effectiveness, potentially creating a need for new vaccines.
- Data limitations. According to FDA, as with all licensed vaccines, there can be limitations in safety data obtained from pre-licensure clinical trials of a COVID-19 vaccine. For example, the clinical trials may not be large enough to detect rare adverse reactions.
- Manufacturing constraints. A shortage of manufacturing capacity may lead to production bottlenecks. Manufacturing supply chains have been strained by global demand for certain vaccine ingredients and equipment during the COVID-19 pandemic. In addition, personnel with specialized skills are needed to run manufacturing facilities.
Policy Context and Questions
- What mechanisms are most effective for quickly scaling up vaccine manufacturing, production, and distribution?
- What could improve the scientific understanding of unknowns such as how long vaccines protect people, and how safe and effective they are in certain populations, such as young children?
For more information, contact Karen Howard at 202-512-6888 or HowardK@gao.gov.
