VA Health Care: Improvements in Oversight Needed for Reusable Medical Equipment
Fast Facts
Reusable medical equipment (such as flexible, camera-bearing endoscopes) must be reprocessed between uses—that is, it must be cleaned, disinfected, or sterilized. The Veterans Health Administration has rules for such steps and oversees the process at VA medical centers.
We testified that VHA did not have reprocessing inspection results for all its facilities, so it can't ensure that its medical centers are following its rules. VHA also doesn't know whether it has enough staff for its reprocessing programs.
We've recommended that VHA take steps to ensure that these inspections occur, and that the agency examine its staffing needs.
An endoscope is a kind of reusable medical equipment
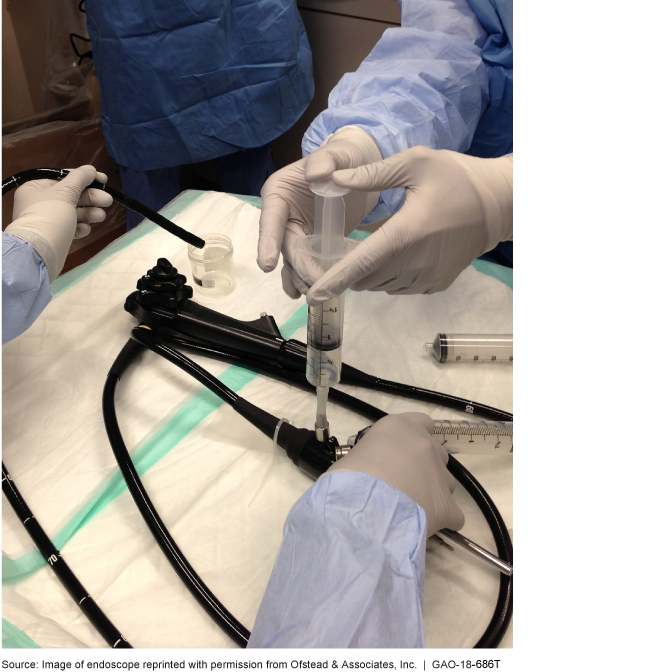
This is a photo of clinicians using an endoscope
Highlights
What GAO Found
GAO found that the Department of Veterans Affairs' (VA) Veterans Health Administration (VHA) does not have reasonable assurance that VA Medical Centers (VAMC) are following policies related to reprocessing reusable medical equipment (RME). Reprocessing involves cleaning, sterilizing, and storing surgical instruments and other RME, such as endoscopes. VHA has not ensured that all VAMCs' RME inspections have been conducted because it has incomplete information from the annual inspections by Veterans Integrated Service Networks (VISN), which oversee VAMCs. For fiscal year 2017, VHA did not have 39 of the 144 VISN reports from the VISNs' inspections of their VAMCs' Sterile Processing Services departments. VISNs were able to provide GAO with evidence that they had conducted 27 of the 39 missing inspections; top areas of non-adherence in these inspections were related to quality and training, among other things. Although VHA has ultimate oversight responsibility, a VHA official told GAO that VHA had not been aware it lacked complete inspection results because it has largely relied on the VISNs to ensure complete inspection result reporting. Without analyzing and sharing complete information from inspections, VHA does not have assurance that its VAMCs are following RME policies designed to ensure that veterans receive safe care.
An endoscope is a kind of reusable medical equipment

GAO also found that VAMCs face challenges operating their Sterile Processing Services programs--notably, addressing workforce needs. Almost all of the officials from all 18 VISNs and selected VAMCs GAO interviewed reported Sterile Processing Services workforce challenges, such as lengthy hiring timeframes and limited pay and professional growth potential. According to officials, these challenges result in programs having difficulty maintaining sufficient staffing. VHA officials told GAO that the office is considering studying Sterile Processing Services staffing at VAMCs, although VHA does not have definitive plans to do so. VHA's Sterile Processing Services workforce challenges pose a potential risk to VAMCs' ability to ensure access to sterilized medical equipment, and VHA's failure to address this risk is inconsistent with standards for internal control in the federal government. Until VHA examines these workforce needs, VHA won't know whether or to what extent the reported challenges adversely affect VAMCs' ability to effectively operate their Sterile Processing Services programs and ensure access to safe care for veterans.
Why GAO Did This Study
This testimony summarizes the information contained in GAO's August 2018 report, entitled VA Health Care: Improved Oversight Needed for Reusable Medical Equipment (GAO-18-474).
For more information, contact Sharon Silas at (202) 512-7114 or silass@gao.gov.
