VA Health Care: Improved Oversight Needed for Reusable Medical Equipment
Fast Facts
Reusable medical equipment, such as a flexible, camera-bearing endoscope, must be reprocessed between uses. It must be cleaned, disinfected or sterilized. The Veterans Health Administration has rules for such steps and oversees the process in VA Medical Centers.
We found VHA did not have complete reprocessing inspection results and can't ensure that its medical centers are following its rules. We also found VHA does not know whether its current workforce is adequate to operate reprocessing programs and ensure access to safe care for veterans.
We recommended that VHA take steps to ensure inspections occur and that it examine workforce needs.
An endoscope is a kind of reusable medical equipment
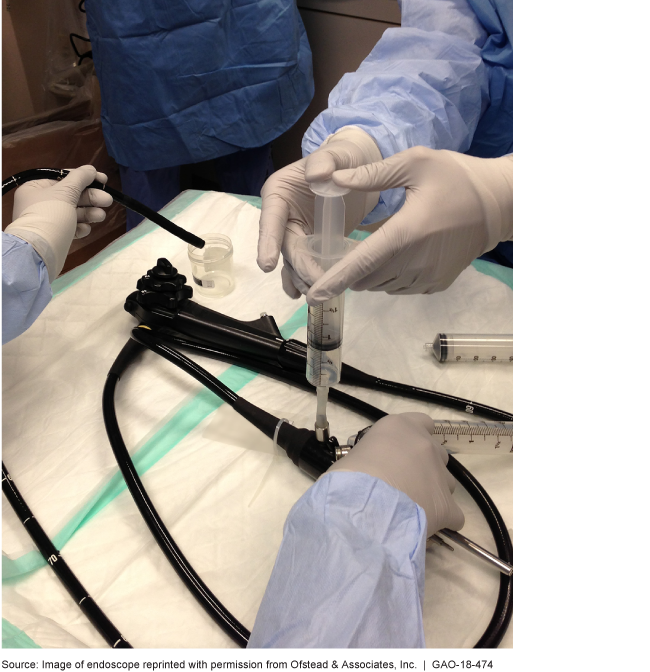
This is a photo of clinicians using an endoscope.
Highlights
What GAO Found
GAO found that the Department of Veterans Affairs' (VA) Veterans Health Administration (VHA) does not have reasonable assurance that VA Medical Centers (VAMC) are following policies related to reprocessing reusable medical equipment (RME). Reprocessing involves cleaning, sterilizing, and storing surgical instruments and other RME, such as endoscopes. VHA has not ensured that all VAMCs' RME inspections have been conducted because it has incomplete information from the annual inspections by Veterans Integrated Service Networks (VISN), which oversee VAMCs. For fiscal year 2017, VHA did not have 39 of the 144 VISN reports from the VISNs' inspections of their VAMCs' Sterile Processing Services departments. VISNs were able to provide GAO with evidence that they had conducted 27 of the 39 missing inspections; top areas of non-adherence in these inspections were related to quality and training, among other things. Although VHA has ultimate oversight responsibility, a VHA official told GAO that VHA had not been aware it lacked complete inspection results because it has largely relied on the VISNs to ensure complete inspection result reporting. Without analyzing and sharing complete information from inspections, VHA does not have assurance that its VAMCs are following RME policies designed to ensure that veterans receive safe care.
An endoscope is a kind of reusable medical equipment

GAO also found that VAMCs face challenges operating their Sterile Processing Services programs—notably, addressing workforce needs. Almost all of the officials from all 18 VISNs and selected VAMCs GAO interviewed reported Sterile Processing Services workforce challenges, such as lengthy hiring timeframes and limited pay and professional growth potential. According to officials, these challenges result in programs having difficulty maintaining sufficient staffing. VHA officials told GAO that the office is considering studying Sterile Processing Services staffing at VAMCs, although VHA does not have definitive plans to do so. VHA's Sterile Processing Services workforce challenges pose a potential risk to VAMCs' ability to ensure access to sterilized medical equipment, and VHA's failure to address this risk is inconsistent with standards for internal control in the federal government. Until VHA examines these workforce needs, VHA won't know whether or to what extent the reported challenges adversely affect VAMCs' ability to effectively operate their Sterile Processing Services programs and ensure access to safe care for veterans.
Why GAO Did This Study
VHA operates one of the largest health care delivery systems in the nation, serving over 9 million enrolled veterans. In providing health care services to veterans, VAMCs use RME which must be reprocessed—that is, cleaned, disinfected, or sterilized—between uses. Improper reprocessing of RME can negatively affect patient care. To help ensure the safety of veterans, VHA policy establishes requirements VAMCs must follow when reprocessing RME and requires a number of related oversight efforts.
GAO was asked to review VHA's reprocessing of RME. This report examines (1) VHA's oversight of VAMCs' adherence to RME policies and (2) challenges VAMCs face in operating their Sterile Processing Services programs, and any efforts by VHA to address these challenges. GAO reviewed relevant VHA documents including RME policies and VISN inspection results for fiscal year 2017. GAO interviewed officials from VHA, all 18 VISNs, and four VAMCs, selected based on geographic variation, VAMC complexity, and data on operating room delays. GAO examined VHA's oversight in the context of federal internal control standards on communication, monitoring, and information.
Recommendations
GAO is making three recommendations to VHA, including that it ensure all RME inspections are being conducted and complete results reported, and that it examine Sterile Processing Services workforce needs and make adjustments, as appropriate. VA concurred with these recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Veterans Health Administration | The Under Secretary of Health should ensure all RME inspections are being conducted and reported as required and that the inspection results VHA has are complete. (Recommendation 1) |
VA agreed with this recommendation and has provided information on the specific actions it has taken to ensure RME inspections are being conducted, reported, and include complete results. Specifically, based on information provided in February 2021, VA established an oversight process for reviewing and monitoring findings from the inspections that includes an accountability tool (allowing for the standardization of inspections and data reviews, among other capabilities) that is utilized by both the National Program Office of Sterile Processing and a new National Reusable Medical Equipment Advisory Board.
|
| Veterans Health Administration | The Under Secretary of Health should consistently analyze and share top common RME inspection findings and possible solutions with VISNs and VAMCs. (Recommendation 2) |
VA agreed with this recommendation and has provided information on specific actions it has taken to analyze and share RME inspection findings and solutions. For example, VA has created trainings and enhanced communications through which information on top RME findings and potential solutions have been shared. VA has also indicated that it will continue analyzing and sharing RME inspection findings on an ongoing basis.
|
| Veterans Health Administration | The Under Secretary of Health should examine the Sterile Processing Services workforce needs and take action based on this assessment, as appropriate. (Recommendation 3) |
VA agreed with this recommendation and provided regular updates on the work done to assess SPS workforce needs since the report's release in 2018. In June 2023, VA provided documents showing that it had assessed the SPS workforce needs and acted on the findings. The assessment included an analysis of workforce data (e.g., data on gains and losses, vacancy rates, retirement projections) and the documents described how the major actions taken based on the assessment included: a revision of the qualification standard, an enhanced market-based approach to pay, and the establishment of an occupational specific recruitment and development structure. VA documents also indicated it would continue to monitor SPS workforce needs in the future.
|
