Biomedical Research: Actions Needed to Adopt Collaboration Practices to Address Research Duplication
Fast Facts
The federal government has long invested in biomedical research through different Department of Health and Human Services agencies. This report, the first in a series, looks at potential duplication of research efforts across four such agencies.
The agencies have practices in place to avoid unnecessary research duplication. For example, agency staff regularly meet to share priorities and identify duplicative work.
In addition, the newest health research agency's advisory committee is working to avoid unnecessary duplication there by coordinating with other agencies. We recommended finalizing the committee’s charter and defining plans to help.

Highlights
What GAO Found
The Department of Health and Human Services (HHS) has long invested in biomedical research. Within HHS, among others, the Advanced Research Projects Agency for Health (ARPA-H), the National Institutes of Health (NIH), the Biomedical Advanced Research and Development Authority (BARDA), and the Food and Drug Administration (FDA) fund or conduct biomedical research. Each of these four HHS agencies' research activities have the potential for duplication when funding research in common areas.
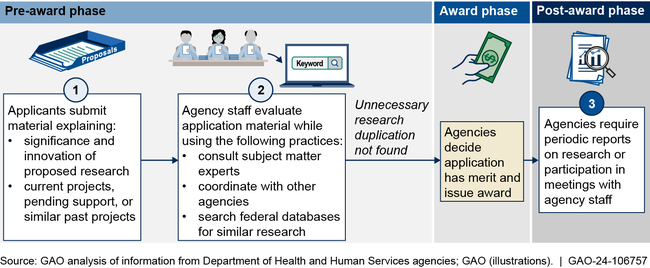
GAO found the four selected HHS agencies use multiple practices intended to help avoid unnecessary research duplication. These include reviewing project and funding information provided by applicants, consulting with experts and other agencies, and using databases to identify potentially overlapping research (see figure). When evaluating instances of research duplication, agency staff also distinguish between necessary and unnecessary duplication. GAO previously reported that some research duplication is necessary to confirm results or otherwise advance a project or field, whereas unnecessary duplication is research not needed to replicate or complement prior results.
Example of HHS Agencies' Practices to Avoid Unnecessary Research Duplication
In 2022, Congress directed the ARPA-H Director to, among other things, coordinate with other federal departments and agencies to ensure that ARPA-H's research is free of unnecessary duplication and established the ARPA-H Interagency Advisory Committee of eight federal agencies to coordinate efforts, among other functions. Such coordination can be a means for ARPA-H and committee member agencies to share information on their research activities and help ARPA-H avoid unnecessary duplication. ARPA-H officials told GAO that the committee will serve as a forum to identify and address potential research duplication between ARPA-H and these agencies. However, the Committee's draft charter does not mention how the members would collaborate to help ARPA-H identify and avoid funding research duplication. Leading practices for interagency collaboration include identifying shared goals and having documented agreements regarding the collaboration. By finalizing the charter to include members' agreement on collaboration methods to avoid ARPA-H funding unnecessary duplication, ARPA-H will be better positioned to improve the future return on the nation's investment in transformational health research.
Why GAO Did This Study
HHS's mission is to enhance the health and well-being of all Americans by, among other things, fostering sound, sustained advances in the sciences. HHS's longstanding agencies—NIH, BARDA, FDA—as well as its newest agency, ARPA-H, fund biomedical research.
The Consolidated Appropriations Act, 2023, includes a provision for GAO to issue a series of reports on potential duplication in HHS's biomedical research and development portfolio. To manage the scope and reporting timeline, this first report focuses on ARPA-H, BARDA, FDA, and NIH as specified in the Act. It (1) describes practices used by the selected HHS agencies to identify and avoid unnecessary research duplication and (2) examines ARPA-H's collaboration and efforts to establish an interagency advisory committee as a potential means to prevent unnecessary research duplication.
To conduct this work, GAO reviewed agency information, and legislation, among other documents. GAO also interviewed agency officials and a non-generalizable selection of non-federal experts in biomedical research.
Recommendations
GAO recommends that ARPA-H finalize the Interagency Advisory Committee charter to clearly define how the participating members agree to share information to avoid ARPA-H's unnecessary research duplication with that of HHS and other federal agencies. HHS neither agreed nor disagreed with the recommendation. GAO maintains the recommendation is warranted.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Advanced Research Projects Agency for Health | The Director of ARPA-H should finalize the ARPA-H Interagency Advisory Committee's charter to clearly define how the participating members agree to share information to avoid ARPA-H's unnecessary research duplication with that of HHS and other federal agencies. |
In September 2024, ARPA-H provided documentation that the participating members agreed to the finalized Interagency Advisory Committee Charter. By finalizing the charter to include members' agreement on collaboration methods to avoid ARPA-H funding unnecessary duplication, ARPA-H is better positioned to improve the future return on the nation's investment in health research.
|
