Generic Drug Applications: FDA Should Take Additional Steps to Address Factors That May Affect Approval Rates in the First Review Cycle
Fast Facts
Companies that make generic drugs—which have the same active ingredients as brand-name drugs—have to apply for FDA approval to market them.
After reviewing an application, FDA may return it with comments. The company can address the comments and resubmit. On average, applications go through 3 of these review cycles before approval (which may take years).
We found FDA approved 12% of generic drug applications in the first cycle in 2015-2017.
FDA took steps to try to increase first-cycle approvals, but we found cases where FDA’s comments were unclear and potentially hard to address.
We made recommendations to help FDA address those concerns.

Photo of an FDA sign marking headquarters.
Highlights
What GAO Found
GAO found that 12 percent of the 2,030 generic drug applications reviewed by the Food and Drug Administration (FDA) from fiscal years 2015 through 2017 were approved in the first review cycle. The first review cycle begins when FDA accepts a generic drug application for review and ends when FDA makes its first decision about whether the drug should be approved for marketing and sale. For applications that were not approved in that first cycle, the application must undergo one or more subsequent review cycles to obtain approval, delaying the generic drug's arrival to market.
Number and Percentage of Generic Drug Applications Approved in the First Review Cycle, Fiscal Years 2015–2017
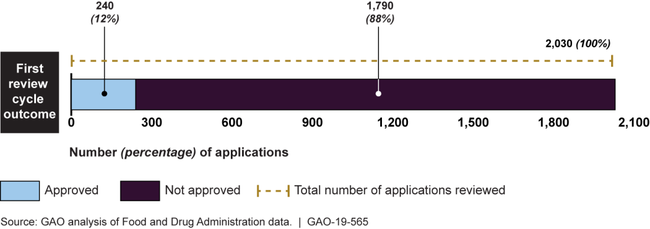
GAO identified several factors that may have contributed to whether a generic drug was approved during the first review cycle. For example, certain types of complex drugs were less likely to receive approval in the first review cycle, such as eye drops or other drugs administered through the eye.
FDA has taken steps to increase the rate of generic drug approvals in the first review cycle. For example, FDA has increased communication with applicants and introduced templates for reviewers to improve the consistency and clarity of their comments. However, GAO's review of a judgmental selection of 35 applications found examples of variation in the clarity and content of FDA's comments to applicants. Such variation may have contributed to whether applicants could adequately address deficiencies within the first cycle, and therefore whether the applications were approved. In addition, stakeholders GAO interviewed expressed concern that changes to the brand-name drug's labeling mid-cycle could delay or prevent generic drugs' approval in the first review cycle, and some stakeholders said they believe that the labeling changes may be strategically timed to delay approvals. Although FDA officials noted that it would be difficult for brand-name companies to time labeling changes in this way, they said that the agency has not conducted analysis that would enable it to assess the validity of these concerns. Therefore, FDA lacks the information needed to respond to these concerns or address problems should they exist.
Why GAO Did This Study
Generic drugs—copies of brand-name drugs—lead to significant cost savings. Before a generic drug can be marketed, FDA must approve the generic drug application. According to FDA, applications go through an average of three cycles of review before being approved, which may take years.
The FDA Reauthorization Act of 2017 included a provision for GAO to study issues regarding the approval of generic drug applications in the first review cycle. This report examines 1) the first review cycle approval rate of generic drug applications in recent years and factors that may have contributed to whether applications were approved; and 2) changes FDA has made to increase the first review cycle approval rate. GAO reviewed FDA data on all generic drug applications reviewed from fiscal years 2015 through 2017 and documentation from the first review cycle for a judgmental selection of 35 applications from fiscal years 2017 and 2018. GAO also interviewed a non-generalizable selection of stakeholders. Applications and stakeholders were chosen to ensure variation in experience with the approval process.
Recommendations
GAO recommends that FDA 1) take additional steps to address inconsistency in its written comments to generic drug application sponsors and 2) assess the extent to which the timing of brand-name drug companies' drug labeling changes affects the approval of generic drugs and take steps, as appropriate, to limit the effect. HHS concurred with GAO's recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration | The Commissioner of FDA should take additional steps to address inconsistency in its written comments to generic drug applicants—including the clarity of writing and the content of comments—among reviewers, such as requiring additional training for reviewers. (Recommendation 1) |
FDA concurred with the recommendation and has taken steps to implement it. As of May 2022, FDA has taken various steps to improve consistency in written comments to generic drug applicants, including the clarity of writing and content of comments, and stated that the agency implemented enhanced processes, templates, training, and other tools to improve the clarity and content of written comments. Specifically, FDA developed and revised letter and review templates, drafted internal standard operating procedures and reviewer guides to enhance consistency in communications about application deficiencies, compiled knowledge databases to reinforce subject matter expertise, and trained staff in the resources available to improve the clarity and content of written responses to generic drug application sponsors.
|
| Food and Drug Administration | The Commissioner of FDA should assess the extent to which the timing of brand-name drug companies' drug labeling changes affect the approval of generic drug applications in the first review cycle, and take steps, as appropriate, to limit the effect of brand-name drug labeling changes on pending generic drug applications. (Recommendation 2) |
FDA concurred with this recommendation and has taken steps to implement it by limiting the effect of brand-name drug labeling changes on pending generic drug applications' approvals. Specifically, as of March 2023, FDA stated that in cases where a generic drug application would likely be approvable if not for a difference in labeling due to a brand-name drug labeling change, FDA reviewers will keep the application open for a period of time up to 60 days (rather than denying it) to provide the applicant the opportunity to revise the labeling. This practice has been included in FDA's commitment letter for the reauthorization of the Generic Drug User Fee Amendments for fiscal years 2023-2027 (GDUFA III). In addition, the Consolidated Appropriations Act, 2023 amended section 505(j)(10)(A) of the Federal Food, Drug, and Cosmetic Act to provide that generic drug applications with labeling changes are eligible for approval when FDA has approved a change to the brand-name drug's labeling within the previous 90 days, rather than 60 days. This would allow a generic drug application to be approved with certain labeling differences if there is a recent brand-name labeling change, as long as the generic drug applicant provides revised labeling within 60 days of approval.
|
