Medicare: CMS's Round 2 Durable Medical Equipment and National Mail-order Diabetes Testing Supplies Competitive Bidding Programs
Fast Facts
Medicare is changing how it determines how much to pay for certain durable medical equipment items, such as oxygen, wheelchairs, and walkers. These ongoing changes helps Medicare (and its beneficiaries) pay less for these items—saving Medicare about $3.6 billion between mid-2013 and 2015, according to its estimates. It also limits the number of suppliers that are eligible to furnish these items to Medicare beneficiaries. Our report takes a look at the effects of the change on beneficiaries' access to and use of the relevant items.
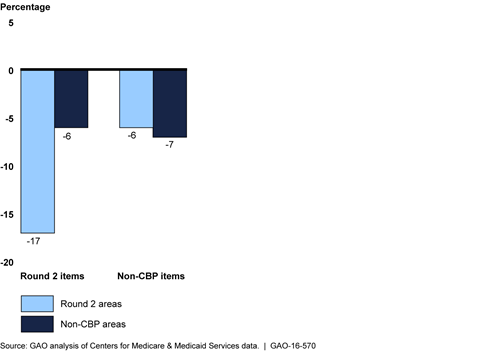
Percentage Change from 2012 to 2014 in Number of Beneficiaries Receiving Durable Medical Equipment Items, by Whether Item or Area Was Included in Competitive Bidding Program (CBP) Round 2
Bar chart showing changes in utilization of durable medical equipment.
Highlights
What GAO Found
The number of beneficiaries receiving durable medical equipment (DME) items covered under the competitive bidding program (CBP) generally decreased after implementation of two CBP phases that began July 1, 2013—round 2 and the national mail-order program for diabetes testing supplies. Under the CBP, (administered by the Centers for Medicare & Medicaid Services (CMS)), only competitively selected contract suppliers can furnish certain DME items at competitively determined prices to beneficiaries in designated competitive bidding areas. From the year before (2012) to the year after (2014) implementation, the number of beneficiaries receiving covered items in round 2 areas decreased 17 percent, compared with a 6 percent decrease for beneficiaries in non-CBP areas. The number of beneficiaries that received diabetes testing supplies through the national mail-order program also decreased 39 percent between 2012 and 2014, with a corresponding 13 percent increase in the number of beneficiaries receiving these items through retail outlets. CMS officials stated that CBP has helped limit fraud and abuse and may have curbed unnecessary utilization of some CBP-covered items in competitive bidding areas.
CMS reports that available evidence from the agency's monitoring efforts indicates that the implementation of round 2 and the national mail-order program have had no widespread effects on beneficiary access. In particular, CMS has reported that its health status monitoring tool has not detected any changes in health measures attributable to the CBP, and the results of its 2014 post-CBP beneficiary satisfaction surveys remained positive. In addition, the number of CBP inquiries and complaints generally decreased throughout the first 2 years of round 2 and the national mail-order program. CMS officials told GAO that CMS took measures to ensure that contract suppliers met their contract obligations, such as investigating complaints using secret shopping calls, and terminating contracts of suppliers that remained noncompliant after receiving targeted education. However, some beneficiary advocacy groups and state hospital associations reported specific access issues, such as difficulty locating contract suppliers that will furnish certain items and delays in delivery of DME items.
Round 2 and the national mail-order program included 801 separate competitive bidding area and product category competitions. Most of these competitions had at least five active contract suppliers in 2014. However, 11 percent of the competitions had three or fewer active contract suppliers and 1 percent had just one active contract supplier. In addition, while multiple suppliers had substantial shares of the market for most competitions, in some competitions a single supplier had a majority. For example, in 6 percent of the competitions, one contract supplier had at least 90 percent of the market. Conversely, 11 percent of contract suppliers did not furnish any CBP-covered items for any competitions in their contract. CMS officials told GAO that CMS monitors these suppliers to help ensure that they are meeting their contractual obligations, such as being willing to service all beneficiaries in their areas and to furnish the same items to Medicare beneficiaries that they make available to other customers.
The Department of Health and Human Services provided technical comments on a draft of this report, which were incorporated as appropriate.
Why GAO Did This Study
To achieve Medicare savings for DME, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 required that CMS implement the CBP for certain DME, such as wheelchairs and oxygen, in phases, or rounds. Round 1 started in 2008, and round 2 and the national mail-order program started in 2013. CMS estimated that the first 2 years of round 2 and the national mail-order program saved Medicare approximately $3.6 billion. GAO has reported on several prior CBP rounds.
GAO was asked to continue to review the implementation of the CBP. In this report, GAO examines the extent to which round 2 and the national mail-order program have affected (1) utilization of CBP-covered DME items, and (2) beneficiaries' access to DME items. This report also (3) describes the number and market shares of the round 2 and mail-order program suppliers.
To examine the effect of CBP on utilization, GAO used Medicare DME claims data from 2012 and 2014—the year before and the year after implementation of round 2—to compare the number of beneficiaries who received CBP-covered DME items. To examine the effect of CBP on beneficiary access, GAO reviewed information about CMS's efforts to monitor the effects of the CBP, and interviewed selected Medicare beneficiary organizations and state hospital associations. To describe the supplier markets, GAO analyzed 2014 Medicare claims data, the latest year with complete available data when GAO began this engagement.
For more information, contact Kathleen M. King at (202) 512-7114 or kingk@gao.gov.
