Drug Shortages: Threat to Public Health Persists, Despite Actions to Help Maintain Product Availability
Highlights
What GAO Found
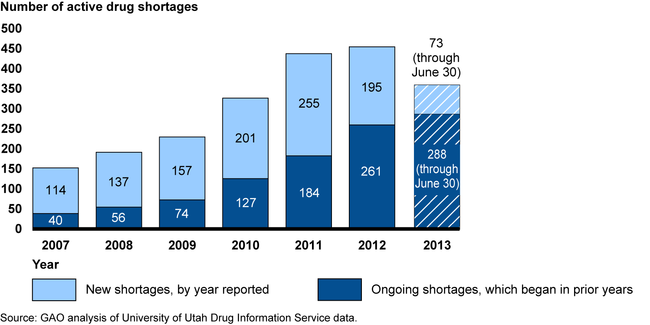
The number of drug shortages remains high. Although reports of new drug shortages declined in 2012, the total number of shortages active during a given year—including both new shortages reported and ongoing shortages that began in a prior year—has increased since 2007. Many shortages are of generic sterile injectable drugs. Provider association representatives reported that drug shortages may force providers to ration care or face difficulties finding alternative drugs.
Number of Active Drug Shortages from January 2007 through June 2013
The immediate cause of drug shortages can generally be traced to a manufacturer halting or slowing production to address quality problems, triggering a supply disruption. Other manufacturers have a limited ability to respond to supply disruptions due to constrained manufacturing capacity. GAO also identified potential underlying causes specific to the economics of the generic sterile injectable drug market, including that low profit margins have limited infrastructure investments or led some manufacturers to exit the market.
While shortages have persisted, the Food and Drug Administration (FDA) has prevented more potential shortages in the last 2 years by improving its responsiveness. FDA has initiated steps to improve its response to shortages, such as modifying the information on its drug shortages website. However, there are shortcomings in its management of drug shortage data that are inconsistent with federal internal control standards. For example, FDA has not created policies or procedures governing the management of the data and has not conducted routine analyses using these data. Such shortcomings could ultimately hinder FDA's efforts to understand the causes of specific shortages as well as undermine its efforts to prevent them from occurring.
Why GAO Did This Study
From prolonged duration of a disease, to permanent injury, to death, drug shortages have led to harmful patient outcomes. FDA—an agency within the Department of Health and Human Services (HHS)—works to prevent, alleviate, and resolve shortages. In 2011, GAO recommended that FDA should enhance its ability to respond to shortages. In 2012, the Food and Drug Administration Safety and Innovation Act (FDASIA) gave FDA new authorities to address drug shortages. FDASIA also mandated GAO to study drug shortages.
In this statement, and in the report on which it is based, GAO focuses on (1) trends in recent drug shortages and describes what is known about their effect on patients and providers; (2) the causes of drug shortages; and (3) the progress FDA has made in addressing drug shortages. GAO analyzed data from FDA and the University of Utah Drug Information Service, which is generally regarded as the most comprehensive source of drug shortage information for the time period we reviewed. GAO interviewed officials from FDA, organizations representing providers, and drug manufacturers. GAO also reviewed the literature, relevant statutes, regulations, and documents.
Recommendations
GAO recommended that FDA strengthen internal controls over its drug shortage data and conduct periodic analyses to routinely and systematically assess drug shortage information, using this information to proactively identify drug shortage risk factors. HHS agreed with GAO's recommendations.
