Medical Device Ads are Everywhere—Who’s Monitoring Them for False or Misleading Claims?
You’ve seen the commercials—an easier way to check your blood pressure, your cardiac health, your glucose levels. Advertisements for medical devices are everywhere—TV, print, the internet, and social media platforms. But how are these advertisements monitored for false or misleading claims?
Today’s WatchBlog post looks at our new report on this oversight.
Image

Who monitors ads for false or misleading claims and how?
Millions of medical devices—ranging from knee replacement implants to contact lenses—are advertised on TV, in newspapers and magazines, and online. Oversight of these advertisements are the responsibility of two federal agencies: the Food and Drug Administration (FDA) and the Federal Trade Commission (FTC).
FDA. You may already know that the FDA is responsible for ensuring that many products are safe to use before they enter the market. But you may not know that FDA is also responsible for monitoring how some of these products are advertised. Specifically, FDA is responsible for monitoring restricted medical devices (those that can only be sold under certain conditions to assure their safety and effectiveness), such as cardiac pacemakers and heart valves.
FTC. The FTC primarily oversees advertisements for devices that are not restricted by FDA. This includes most over-the-counter medical devices such as pregnancy tests and some prescription devices such as contact lenses.
Image
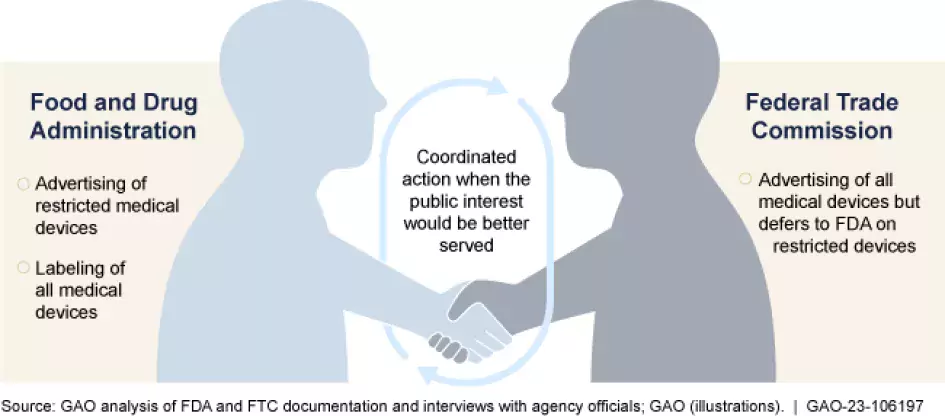
Tracking the false or misleading advertising is a difficult task. With millions of devices on the market, officials from both agencies told us they lack the resources to monitor all advertisements. As a result, both agencies told us that they prioritize oversight of devices that could potentially cause the most harm or injury (whether financial or physical) when determining how to allocate their resources.
How often is action taken against false claims and what is the penalty?
FDA and FTC took more than 300 enforcement actions related to medical device advertisements between 2018 and 2022. Enforcement actions can include the following:
- Warning letters: In some circumstances, both agencies will coordinate on enforcement actions, including the issuance of joint warning letters. For example, during the COVID-19 pandemic, the agencies issued a warning letter to a company that made claims that their ceramic-grade magnets could treat or cure the disease.
- Fines: In certain cases, FDA and FTC may fine a business that has violated the agencies’ laws and regulations.
- Prosecution: After an investigation, FDA and FTC may pursue prosecution.
When choosing what actions to take, both FDA and FTC officials said they consider several factors on a case-by-case basis. For example, the level of potential harm or injury that could be caused to consumers.
Monitoring the accuracy of medical device advertisements is an incredibly important job for both the FDA and FTC. Moreover, since 2009, we have placed FDA’s oversight of medical products—including medical devices—on GAO’s High Risk List.
You can help the FDA and FTC to identify unsafe products or those that have been falsely advertised. If you have a concern about a false or misleading ad for a medical device, you can report it to FDA here and here. Fraud can be reported to the FTC here.
Learn more about FDA and FTC efforts to monitor false or misleading medical device ads by checking out our new report.
- Comments on GAO’s WatchBlog? Contact blog@gao.gov.
GAO Contacts
Related Products

GAO's mission is to provide Congress with fact-based, nonpartisan information that can help improve federal government performance and ensure accountability for the benefit of the American people. GAO launched its WatchBlog in January, 2014, as part of its continuing effort to reach its audiences—Congress and the American people—where they are currently looking for information.
The blog format allows GAO to provide a little more context about its work than it can offer on its other social media platforms. Posts will tie GAO work to current events and the news; show how GAO’s work is affecting agencies or legislation; highlight reports, testimonies, and issue areas where GAO does work; and provide information about GAO itself, among other things.
Please send any feedback on GAO's WatchBlog to blog@gao.gov.

